Medical Device Affairs Outsourcing Market Research, 2031
The global medical device affairs outsourcing market was valued at $4.6 billion in 2021, and is projected to reach $8.1 billion by 2031, growing at a CAGR of 6% from 2022 to 2031. Medical device affairs outsourcing may vary in extent from stand-alone service to full-scope solution, length (project-based or long-term partnership with selected vendor), and model (insourcing a dedicated expert for a specific project or functional service provision). Regardless of the approach, various companies tend to look for outsourcing solutions to decrease the number of in-house staff or prioritize strategic projects for the internal resources, optimize the workload of the existing team, centralize particular functions, and increase its oversight. Moreover, there is a clear trend toward outsourcing local regulatory support in smaller or less attractive and financially beneficial markets while keeping all the activities related to high-importance countries for in-house management. The outsourcing vendor management is more likely to drive process standardization & consistency, increasing transparency and effective governance. Moreover, it seems evident that the volumes of regulatory outsourcing are expected to continue to grow stably in the upcoming years as regulatory requirements have become more and more complex.
The growth of the medical device affairs outsourcing market size is driven by increase in number of on-going R&D activities, surge in number of clinical studies conducted, and rise in demand for outsourcing regulatory affairs services.

Furthermore, the medical device regulatory affairs outsourcing helps to augment the volume of product registrations and clinical trial approvals, which contributes toward the medical device affairs outsourcing market growth. However, high cost of clinical trials & product development and change in the regulations regarding medical device in different regions negatively impact the medical device affairs outsourcing industry,. Conversely, the economic growth in emerging market offers the medical device affairs outsourcing market opportunity.
Impact of COVID-19 Pandemic on Medical Device Affairs Outsourcing Market Size
Coronavirus (COVID-19) was discovered in late December, 2019 in Hubei province of Wuhan city in China. The situation of COVID-19 had varied outcome when related to vaccinations. In addition, there is an emerging gap in the economic recovery between high-income and low & middle income countries. After the pandemic severely disrupted global trade, the world witnessed a robust rebound, which helped with the recovery in the year 2021. Trade contributes to speeding up economic recovery from the pandemic by providing sustained foreign demand for exports and ensuring the availability of imported intermediate products and services. The impact of COVID-19 remains negative on medical device affairs outsourcing industry, as medical device companies are finding it difficult to make informed decisions about their products, supply chains, and regulatory obligations in the midst of uncertainty. Medical device professionals have the unenviable task of asking for a pause amid the panic.
For instance, the impact of COVID-19 on clinical trials has been hampered, due to challenges posed by travel bans, hospital and clinic visit restrictions, and social distancing precautions, just to name a few. These factors have translated into multiple issues that pose challenges related to corporate milestones, budgets, and data integrity. IRB meetings and processes are not impacted by any restrictions on travel. As a standard practice, the IRB meets remotely via video conference technology. IRB has been prioritizing the review of the numerous COVID-19 protocols received as well as amendments relating to changes in execution of research due to unforeseen circumstances. Clinical trial protocols are required to be changed to eliminate apparent immediate hazards to participants such as change in-person visits to virtual visits, elimination of study visits or procedures that do not impact the integrity of the study or participant safety, incorporation of screening questions to identify potential COVID-19 exposure.
Medical Device Affairs Outsourcing Market Segmentation
The medical device affairs outsourcing market is segmented into Service, Software and End User. By service, the market is classified into regulatory writing and submissions, regulatory registration services, regulatory consulting and others. By software, the market is bifurcated into cloud-based software and on-premise software. By end user, the market is categorized into pharmaceutical companies, medical technology companies and others. Furthermore, the pharmaceutical companies segment is sub classified into large and medium. The medical technology companies segment is sub segmented into large and medium. Region wise, the market is studied across North America (U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and rest of Europe), Asia-Pacific (China, Japan, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
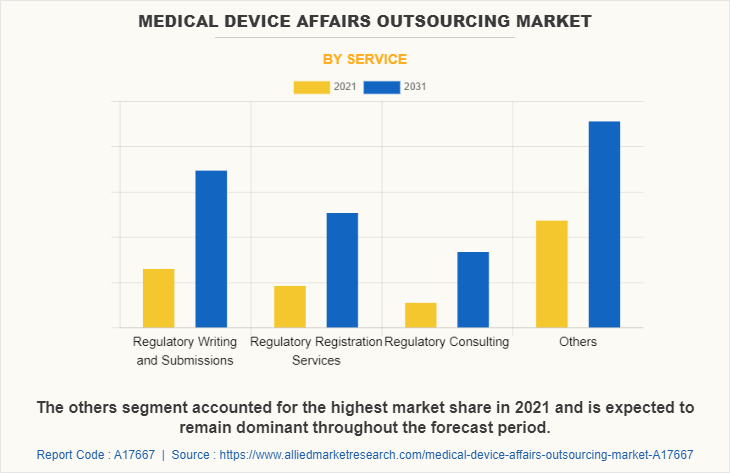
Market By Service Segment Review
By service, the others segment is anticipated to grow with the largest medical device affairs outsourcing market share during the forecast period. This is attributed to the increase in the use of regulatory affairs outsourcing service in the clinical research organization. Recent increase in the demand of the regulatory services have contributed to improve the preference for these services, thus driving the market growth. On the other side, the regulatory writing and submissions is projected to exhibit the fastest market growth during the forecast period due to its increased number of benefits for the medical technology companies.
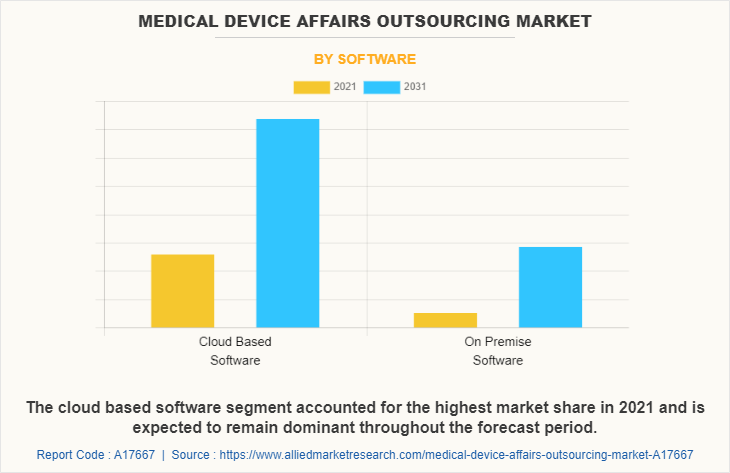
Market By Software Segment Review
By software, the cloud based software segment acquired the major medical device affairs outsourcing market share in 2021, and is anticipated to continue this trend during the forecast period, due to increase in the advantages of the cloud based software. In addition, the cloud based software segment is also expected to exhibit the fastest market growth during the forecast period due to increase in research and development activities regarding medical device.
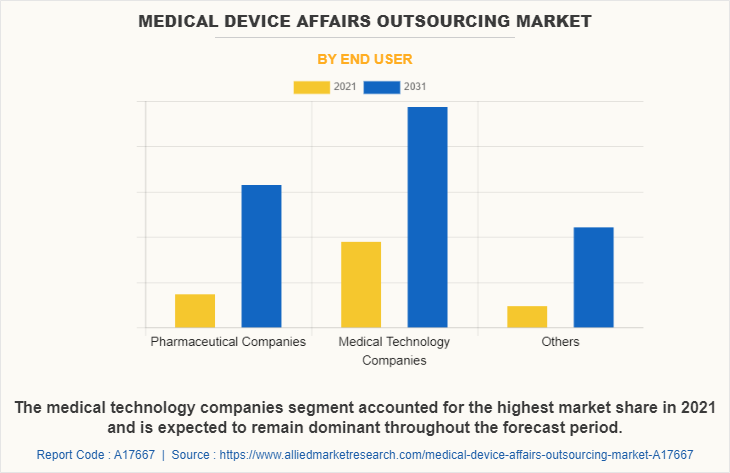
Market By End User Segment Review
By end user, the medical technology companies segment acquired the largest share in 2021, and is anticipated to continue this trend during the forecast period. This is attributed to the increase in number of medical technology companies globally and increase in the number of product development. On the other side, the pharmaceutical companies segment is projected to exhibit the fastest market growth during the forecast period, owing to increase in preference of the market players for using the outsourcing services for the regulatory affairs compliance.
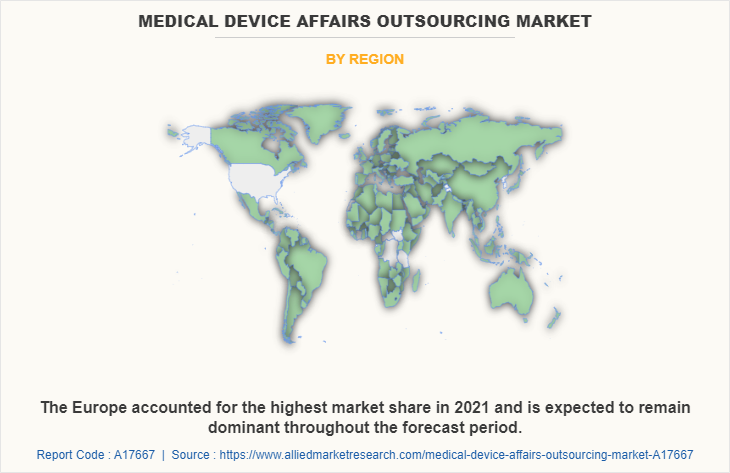
Market By Region Review
Europe accounted for the largest share of revenue in 2021, and is anticipated to maintain its dominance during the medical device affairs outsourcing market forecast period, owing to presence of large number of clinical research organizations, strong presence of key players, increased healthcare expenditure, higher number of research, development, & innovation activities and higher adoption of advanced technologies. Moreover, Asia-Pacific is expected to grow at the highest CAGR, owing to increase in number of clinical trials conducted and rise in awareness related to regulatory affairs services outsourcing benefits.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the medical device affairs outsourcing market analysis from 2021 to 2031 to identify the prevailing medical device affairs outsourcing market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the medical device affairs outsourcing market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global medical device affairs outsourcing market trends, key players, market segments, application areas, and market growth strategies.
Medical Device Affairs Outsourcing Market, by Service Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 8.1 billion |
| Growth Rate | CAGR of 6% |
| Forecast period | 2021 - 2031 |
| Report Pages | 286 |
| By Service |
|
| By Software |
|
| By End User |
|
| By Region |
|
| Key Market Players | Inizio, Charles River, IQVIA Holdings Inc., Wuxi AppTec, Thermo Fisher Scientific, Inc., Indegene Private Limited, Syneos Health, Inc., Icon plc, Parexel International Corporation, Excelya, SGS SA |
Analyst Review
The demand for medical device affairs outsourcing services is expected to witness a significant rise owing to increase in R&D activities and surge in incidences of diseases in both developed & developing economies that require medical device for diagnosis and treatment. Furthermore, the global medical device affairs outsourcing market growth is propelled by surge in number of clinical studies performed, increase in collaboration between market players for development of novel products, and rise in awareness associated with product development.
North America accounted for the highest market share in 2021, due to presence of large number of clinical research organizations, strong presence of key players, increased healthcare expenditure, and heavy expenditure by the government on healthcare, followed by Europe. Moreover, Asia-Pacific is expected to exhibit fastest market growth due to rise in awareness of the regulatory affairs services outsourcing benefits.
The total market value of Medical Device Affairs Outsourcing Market report in 2031 is estimated to be $8,111.02 million.
The forecast period in the market report is from 2022 to 2031
The base year calculated in the Medical Device Affairs Outsourcing Market report is 2021.
The major companies profiled in the report include Excelya, Icon plc, Indegene Private Limited, Inizio (UDG Healthcare plc.), IQVIA Holdings Inc., Parexel International Corporation, SGS SA, Syneos Health, Inc., Thermo Fisher Scientific, Inc. (PPD Inc.), and Wuxi AppTec.
The increase in number of on-going R&D activities, surge in number of clinical studies conducted, and rise in demand for outsourcing regulatory affairs services are the key trends of medical device affairs outsourcing market.
Medical device affairs outsourcing market services assist the original equipment manufacturing (OEM) companies with scalable and flexible solutions that support them to use healthcare personnel resources, resource components, and strategic management.
Europe is the largest regional market for medical device affairs outsourcing.
Yes, the Medical Device Affairs Outsourcing Market report provides PORTER Analysis.
Loading Table Of Content...



