Antivenom Market Research, 2033
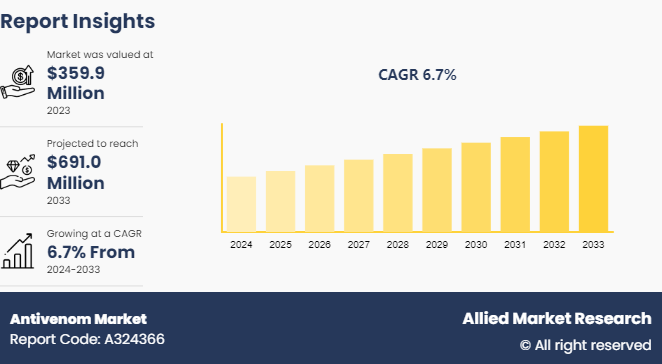
The global antivenom market was valued at $359.9 million in 2023, and is projected to reach $691.0 million by 2033, growing at a CAGR of 6.7% from 2024 to 2033. The rise in venomous animal encounters and research and development efforts for innovative antivenom are expected to propel the market's expansion.
Market Introduction and Definition
Antivenom, also known as antivenin, is a biological product used in the treatment of venomous bites or stings. It is created by extracting antibodies from the plasma of a host animal, such as a horse or sheep, which has been immunized with small, non-lethal doses of venom. These antibodies can then neutralize the effects of venom from various creatures like snakes, spiders, scorpions, and other venomous animals. The antivenom market is a critical segment of the pharmaceutical industry, particularly in regions where venomous bites and stings are common. The market dynamics are influenced by several factors, including the prevalence of venomous species, the availability and effectiveness of antivenom, and the healthcare infrastructure in different regions.
Key Takeaways
- The antivenom market size covers 20 countries. The research includes a segment analysis of each country in terms of value ($Million) for the projected period 2023-2033.
- More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major antivenom industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and assist stakeholders in making educated decisions to achieve their most ambitious growth objectives.
Key Market Dynamics
The antivenom market forecast is influenced by the high incidence of venomous animal encounters, in regions such as parts of Africa, Asia, and Latin America, thus, have a strong demand for antivenom products. Rural and tropical areas, where people are more likely to encounter venomous animals, drive the need for accessible and effective antivenom. Further, government and non-governmental organizations (NGOs) play a significant role in antivenom distribution, especially in rural and underserved areas.
Programs aimed at reducing morbidity and mortality from venomous bites drive market demand. Moreover, innovations in antivenom production, such as recombinant DNA technology and monoclonal antibody-based therapies, are improving the efficacy and safety profiles of antivenom products, thereby boosting antivenom market opportunity. Furthermore, regulatory frameworks and approvals from bodies like the U.S. Food and Drug Administration (FDA) , European Medicines Agency (EMA) , and World Health Organization (WHO) facilitate the development and distribution of antivenom, ensuring quality and accessibility.
Advances in biotechnology, such as recombinant DNA technology and monoclonal antibody therapies, are leading to the development of more effective and safer antivenom products and the same are also upcoming antivenom market trends. Enhancements in the storage and stabilization of antivenom products improve their shelf life and efficacy, making them more accessible in remote areas. Thus, the aforementioned factors are anticipated to drive the market growth.
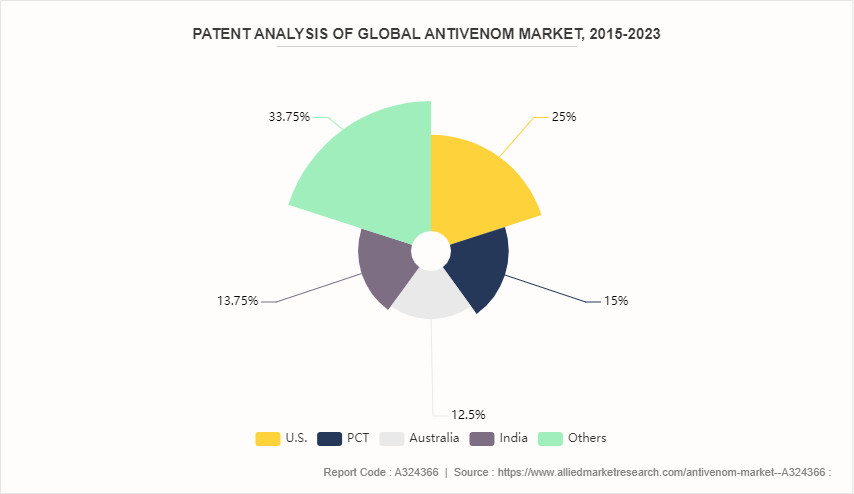
Patent Analysis of Global Antivenom Market, 2015-2023
Although the antivenom market growth has been more rapid in recent years, the industry can draw on the patents filed by various companies. For instance, patents filed by the companies based in U.S. dominated the same with 25% share, Patent Cooperation Treaty (PCT) , holds a share of about 15%, Australia holds a share of about 12.5% and India held a share of about 13.8%.
Market Segmentation
The antivenom market analysis is segmented into type, species, end user, and region. By type, the market is categorized into polyvalent, monovalent, and others. By species, the market is divided into snake, spider, scorpion, and others. By end user, the market is classified into hospitals, clinics, and others. Region-wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional/Country Market Outlook
North America accounted for a major antivenom market share of the antivenom market owing to advanced healthcare infrastructure, robust research and development capabilities, strong regulatory frameworks, and significant investment in public health initiatives. These factors, combined with a strong presence of major key players, contribute to the region’s leading position in the market.
Asia-Pacific antivenom market share is estimated to grow with the highest CAGR during the forecast period, owing to high incidence rates of venomous bites, increasing healthcare investments, and government initiatives. Many regions in Asia-Pacific have large rural and agricultural communities where people are more likely to encounter venomous animals. Companies are focusing on this region to expand their market presence and increase sales.
Industry Trends
- In June 2023, the World Health Organization (WHO) published the first in a series of WHO public-benefit target product profiles (TPPs) for snakebite treatments, to improve the quality of antivenoms available in the market.
- Establishment of local production facilities, and research centers in regions with high demand for antivenom, such as India, Brazil, and parts of Africa, to reduce reliance on imports and ensure a stable supply.
Competitive Landscape
The major players operating in the antivenom market include Boehringer Ingelheim International GmbH, CSL Limited, Merck & Co., Inc., Pfizer Inc., Bharat Serums and Vaccines Ltd., Sanofi Pasteur SA, Instituto Bioclon, Rare Therapeutics, MicroPharm Limited and Haffkine Bio-Pharmaceutical Corporation.
Recent Key Strategies and Developments
- In August 2022, Bharat Serums and Vaccines Ltd. (BSV) announced its collaboration with the ‘Evolutionary Venomics Lab’, at the Indian Institute of Science (IISc) , Bengaluru to develop region-specific antivenoms snakebites in India.
- In April 2021, Rare Disease Therapeutics, Inc. (RDT) announced that the United States Food & Drug Administration (FDA) approved a new expanded indication for ANAVIP (crotalidae immune F (ab’) 2 (equine)) , an equine-derived antivenin, for the management of adult and pediatric patients with North American Pit Viper envenomation.
Key Sources Referred
- National Center for Biotechnology Information
- World Health Organization (WHO)
- National Library of Medicine
- The United States Food and Drug Administration
- U.S. Department of Health & Human Services
- Centers for Disease Control and Prevention
- Eurostat
- Australian Institute of Health and Welfare
- Ministry of Health and Family Welfare
- Fogarty International Center
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the antivenom industry segments, current trends, estimations, and dynamics of the antivenom market by type analysis from 2024 to 2033 to identify the prevailing antivenom market by type opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the antivenom market by type segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global antivenom market size by type trends, key players, market segments, application areas, and market growth strategies.
Antivenom Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 691.0 Million |
| Growth Rate | CAGR of 6.7% |
| Forecast period | 2024 - 2033 |
| Report Pages | 280 |
| By Type |
|
| By Species |
|
| By End User |
|
| By Region |
|
| Key Market Players | Haffkine Bio-Pharmaceutical Corporation, CSL Limited, Sanofi Pasteur SA, Pfizer Inc., MicroPharm Limited, Instituto Bioclon, Bharat Serums and Vaccines Ltd., Rare Therapeutics, Merck & Co., Inc., Boehringer Ingelheim International GmbH |
Increase in the animal bites and R &D activities for their treatments are the upcoming trends of Antivenom Market in the globe.
Polyvalent is the leading type of Antivenom.
North America is the largest regional market for Antivenom Market.
The antivenom market was valued at $359.89 million in 2023.
The major players operating in the antivenom market include Boehringer Ingelheim International GmbH, CSL Limited, Merck & Co., Inc., Pfizer Inc., Bharat Serums and Vaccines Ltd., Sanofi Pasteur SA, Instituto Bioclon, Rare Therapeutics, MicroPharm Limited and Haffkine Bio-Pharmaceutical Corporation.
Loading Table Of Content...



