Autologous Cell Therapy Market Research, 2031
The global autologous cell therapy market was valued at $4.3 billion in 2021, and is projected to reach $29.1 billion by 2031, growing at a CAGR of 21% from 2022 to 2031. Autologous therapy is a process in which patient's own cells or tissues, which are treated outside the body and then reintroduced into the donor. This type of therapy is currently used in orthopedics, modern medicine, aesthetic, and cosmetic dermatology. Various autologous therapies are there such as autologous stem cell-based therapies and autologous immunotherapy that aim to promote wound healing, reduce chronic inflammation, and promote wound closure. Various technologies and fields, such as cellular and molecular biology to virology are used in autologous cell therapy. In heart disease, transplants of autologous blood vessels and arteries have been also used for a long time.

Rise in prevalence of cancer is the key factor that drives the growth of the global autologous cell therapy market. In addition, increase in number of clinical trials and increase in public and private investments for the development of CAR (chimeric antigen receptor)-T therapy in which T cells are collected from a patient are genetically modified ex vivo and re-injected into the patient drive the market growth. However, inflated cost associated with autologous cell therapy and lower treatment facilities may hinder the market growth. In contrast, high growth potential in untapped emerging economics are anticipated to provide lucrative opportunities to the market players.
Factor such as rise in technological advancements, R & D are the upcoming for autologous cell therapy trends the market growth. Furthermore, autologous anti-CD19 CAR T-cell therapy such as Axicabtagene Ciloleucel obtained the FDA approval on 1 April 2022, for the treatment of adults with large B-cell lymphoma. Brexucabtagene autoleucel obtained Food and Drug Administration (FDA) approval in2020, for the treatment of mantle cell lymphoma. These approvals boost the autologous cell therapy market opportunity. Furthermore, these approvals for autologous cell therapy boost the market growth. Slowly progressive and degenerative neurological diseases such as Parkinson's disease (PD) and Alzheimer's disease (AD) elicited the urge for novel therapeutic. Research in last couple of years has shifted toward transplant therapy for these diseases and autologous cell therapy, which is emerging to alleviate symptoms or even reverse disease progression. Thus, the technological advancements for decentralizing manufacturing of autologous cell therapy is anticipated to significantly benefit the autologous cell therapy market forecast.
Impact of COVID-19
The outbreak of COVID-19 has disrupted workflows in the healthcare sector across the world. The disease forced many industries to shut their doors temporarily, including several sub-domains of health care. In addition, decrease in demand for autologous cell therapy, decrease in supply of raw materials and product manufacturing had disrupted due to the closing down of the marketplace this may restrains the market growth. However, COVID-19 pandemic negatively impacted the market on autologous cell therapy. The significant reduction in clinical trials for autologous cell therapy, owing to strict government guidelines against COVID-19 restrains the growth of market. Decline in hospitals visit, closing of borders, and confinement of the population impacted the supply chains of these life-saving medical products in the autologous cell therapy market. In addition, introduction of COVID-19 vaccinations, individuals can now easily access hospitals and cancer treatment facilities, and cancer treatment is given priority, which lead to an increase in demand for autologous cell therapy. Therefore, it is projected that these developments will stabilize the market and drive autologous cell therapy market growth during the forecast period.
Market Segmentation
The autologous cell therapy market is segmented into therapeutic area, end user and region. By therapeutic area, the market is categorized into cancer and others. The cancer segment further includes lymphoma, acute lymphocytic leukemia, and others. By end user, it is classified into hospitals and cancer treatment centers. Region wise, the market is analyzed across North America (U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Latin America and Middle East & Africa).
Segment Review
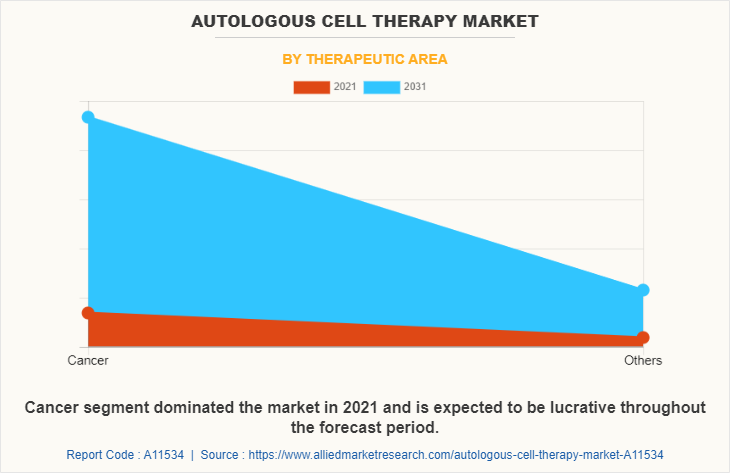
Depending on therapeutic area, the autologous cell therapy market size is segmented into cancer and others. The cancer segment was dominated the global market in 2021 and is remain dominant during the forecast period. The factor which drives the growth of this segment is due to upsurge in the prevalence.
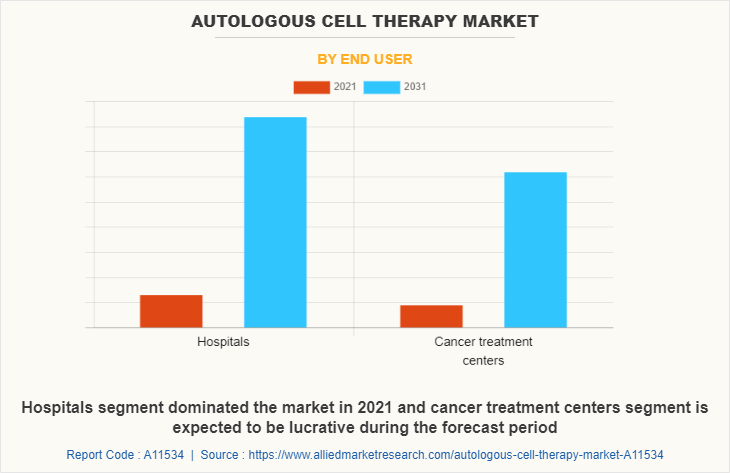
On the basis of end user, the autologous cell therapy market size is segmented into hospitals and cancer treatment centers. The hospitals segment accounted for the largest autologous cell therapy market share revenue in 2021. This was attributed to rise in income, better health awareness and rise in the population associated with cancer diseases. In addition, the availability of cancer treatment in hospital drives the segment growth. However, the cancer treatment centers segment is projected to register the highest CAGR during the forecast period. This is attributed to rise in the number of cancer treatment facilities due to the increase in the prevalence of cancer diseases and centers offering treatment.
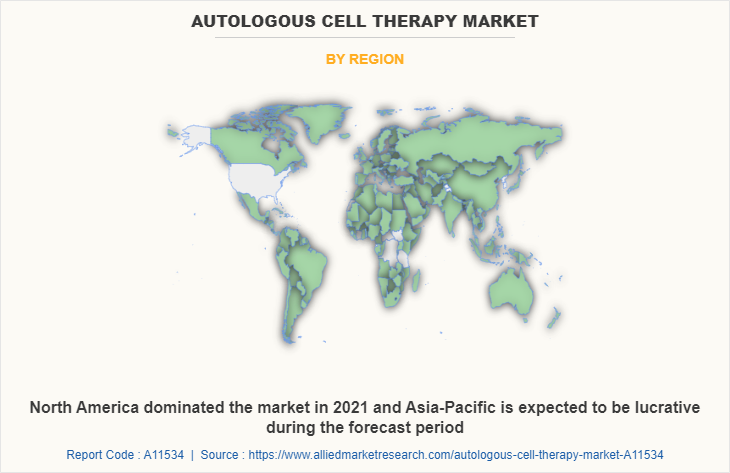
Region wise, North America held a major autologous cell therapy market share, owing to the rise in government initiatives, well-developed healthcare infrastructure, and presence of key players further boost the market growth. However, Asia-Pacific is expected to witness the highest growth rate for the autologous cell therapy market throughout the forecast period with a highest CAGR. The major factors that drive the growth of the autologous cell therapy market in this region is the increase in prevalence of cancer, technological advancements, and increase in number of hospitals and cancer treatment centers.
The key market players profiled in the autologous cell therapy industry include are Bristol Myers Squibb, CORESTEM, Inc., GC Biopharma Corp (GC Cell), Gilead Sciences, Inc. (Kite Pharma, Inc.), Holostem Terapie Avanzate S.r.l., Johnson & Johnson Private Limited (Janssen Biotech, Inc.), Novartis AG, Sanpower Group (Dendreon Pharmaceuticals LLC.), Tegoscience, and Vericel Corporation.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the autologous cell therapy market analysis from 2021 to 2031 to identify the prevailing autologous cell therapy industry opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the autologous cell therapy market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global autologous cell therapy market trends, key players, market segments, application areas, and market growth strategies.
Autologous Cell Therapy Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 29.1 billion |
| Growth Rate | CAGR of 21% |
| Forecast period | 2021 - 2031 |
| Report Pages | 188 |
| By Therapeutic area |
|
| By End user |
|
| By Region |
|
| Key Market Players | Vericel Corporation, Tego Science, Bristol Myers Squibb, CORESTEM, Inc, Johnson & Johnson(Janssen Biotech, Inc), Novartis, Sanpower Group (Dendreon Pharmaceuticals LLC.), Gilead Sciences (Kite Pharma, Inc.), Holostem Terapie Avanzate S.r.l., GC Biopharma (GC Cell) |
Analyst Review
This section provides various opinions of top-level CXOs in the global autologous cell therapy market. According to the insights of CXOs, the global autologous cell therapy market is expected to exhibit high growth potential attributable to factors such as increase in chronic diseases, demand for autologous cell therapy to treat the diseases, and rise in R&D activities for autologous cell therapy.
CXOs further added that rapidly expanding clinical trial activities, commercialization of therapy products, and key players in the market are focusing on adopting strategies to increase accessibility and utilization of autologous cell therapy products in developing economies, which are expected to fuel the market growth during the forecast period. However, higher cost of treatment may hinder the growth of the market.
Furthermore, North America is expected to witness highest growth, in terms of revenue, owing to surge in cases of cancer, robust healthcare infrastructure, presence of key players, and rise in healthcare expenditure. However, Asia-Pacific is anticipated to witness notable growth owing to rise in geriatric population, unmet medical demands, initiatives by government & non-governmental organizations (NGOs) to promote awareness regarding autologous cell therapy, and increase in public–private investments in the healthcare sector.
The growth of global autologous cell therapy market is majorly driven by increase in demand for autologous cell therapy, owing to rise in R&D institutes & organizations for developing autologous cell therapy products and surge in chronic diseases especially cancer.
Cancer is the leading therapeutic area of autologous cell therapy market.
Bristol Myers Squibb, CORESTEM, Inc., GC Biopharma Corp (GC Cell), Gilead Sciences, Inc. (Kite Pharma, Inc.), Holostem Terapie Avanzate S.r.l., Johnson & Johnson Private Limited (Janssen Biotech, Inc), Novartis AG, Sanpower Group (Dendreon Pharmaceuticals LLC.), Tego Science, and Vericel Corporation are the top companies to hold the market share in autologous cell therapy market.
2021 is the base year of autologous cell therapy market.
2022 to 2031 is the forecast period of autologous cell therapy market.
North America is the largest regional market for autologous cell therapy.
The global autologous cell therapy market was valued at $4,307.06 million in 2021, and is projected to reach $29,049.87 million by 2031, registering a CAGR of 21.0% from 2022 to 2031.
Loading Table Of Content...



