Chemotherapy Induced Anemia Market Research, 2032
The global chemotherapy induced anemia market size was valued at $2.7 billion in 2022, and is projected to reach $5.0 billion by 2032, growing at a CAGR of 6.3% from 2023 to 2032. Chemotherapy-induced anemia (CIA) is a common side effect of chemotherapy treatment for cancer. Anemia is a medical condition where the body does not have enough red blood cells to carry oxygen to the tissues. In CIA, chemotherapy drugs can damage the bone marrow, which is responsible for making red blood cells, leading to a decrease in the number of red blood cells in the body. The symptoms of chemotherapy-induced anemia are fatigue, weakness, shortness of breath, dizziness, and pale skin. Treatment for CIA includes blood transfusions, erythropoiesis-stimulating agents (ESAs), and iron supplements.

Market dynamics
Chemotherapy induced anemia market size is driven by an increase in the number of people undergoing chemotherapy, presence of chemotherapy induced anemia industry and the rise in the appearance of anemia in cancer patients. For instance, in June 2020, FibroGen, Inc. a biotechnology research company estimated that about 1.3 million patients undergo chemotherapy every year in the U.S. In addition, as per the same source out of 1.3 million cancer patients undergoing chemotherapy, about 30% to 90% patient develop anemia and its rate increases with each chemotherapy round. Furthermore, with the increase in incidence of cancer, the number of patients receiving chemotherapy is also increasing, which leads to a higher incidence of chemotherapy-induced anemia. In addition, surge in diagnosis of grade 1 anemia in cancer patients also contribute towards the market growth. For instance, according to the National Center for Biotechnology Information (NCBI), anemia is common in cancer patients with incidence rates ranging from 22.7% to 63% in 2020. Chemotherapy is a common treatment for many types of cancer, and the demand for this treatment is increasing due to various factors such as improved cancer screening, early diagnosis, and the development of new chemotherapy drugs.
In addition, growing awareness among healthcare professionals and patients about the importance of managing anemia as part of cancer treatment, also contributes toward the market growth. Furthermore, increase in demand for chemotherapy induced anemia treatments due to availability of erythropoiesis-stimulating agents and iron supplements further boost the chemotherapy induced anemia market growth
The growth of the chemotherapy induced anemia market is expected to be driven by developed countries such as U.S., Canada, and Germany, due to availability of treatment options, rise in geriatric population who are more prone to develop cancer and availability of favorable reimbursement policies that can make CIA treatments affordable for patients. Furthermore, the healthcare industry in emerging economies is developing at a significant rate, owing to a rise in demand for enhanced healthcare services, and an increase in geriatric population.
In addition, rise in technological advancements such as development of biosimilars and other therapies for the treatment of CIA further propel the market growth. For instance, in 2022, the U.S. Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for Reblozyl (luspatercept-aamt) as a first-line treatment of anemia in adults with lower-risk myelodysplastic syndromes (MDS). In addition, the European Medicines Agency (EMA) also validated the application for Reblozyl as a first-line treatment of anemia in adults with lower-risk MDS.
However, the side effects associated with chemotherapy induced anemia therapies restrain the growth of the market. For instance, erythropoiesis-stimulating agents are associated with several side effects such as tumor progression, heart failure, loss of consciousness, and death. In addition, the side effects of blood transfusion are allergic reaction, iron overload, and febrile non-hemolytic transfusion reactions. Furthermore, high cost of chemotherapy induced anemia treatments negatively impacts the market growth.
On the other hand, development of novel therapies with minimum side effects and increase in awareness about early disease detection are expected to create lucrative opportunities for the market growth.
The outbreak of COVID-19 has disrupted workflows in the health care sector around the world. The disease has forced a number of industries to shut their doors temporarily, including several sub-domains of health care. The global chemotherapy induced anemia market experienced a decline during the pandemic due to disruption of cancer care services, including chemotherapy treatments, disruption in supply of pharmaceutical drugs. In addition, the pandemic has also disrupted clinical trials for new CIA treatments, leading to delays in the development and approval of new therapies. On the other hand, the increase in demand for CIA treatments post pandemic and increase in prevalence of cancer and anemia are expected to drive the market growth during the chemotherapy induced anemia market forecast period.
Segmental Overview
The chemotherapy induced anemia market share is segmented into treatment, grade and end user and region. On the basis of grade, the market is classified into grade 1, grade 2, and grade 3 & 4. On the basis of treatment, the market is segmented into blood transfusion, erythropoietin-stimulating agents, iron and other supplements. On the basis of end user, the market is bifurcated into hospitals, cancer centers, and others. The others segment includes cancer research centers, cancer rehabilitation centers, and home use. Region wise, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and rest of Europe), Asia-Pacific (China, Japan, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
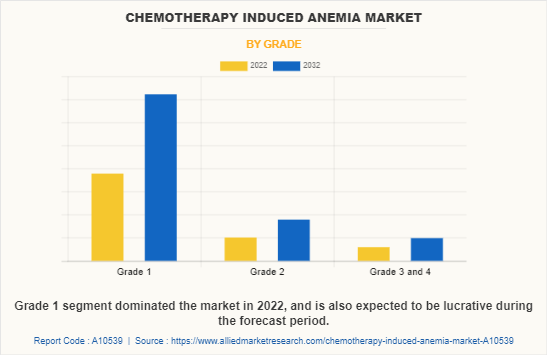
By Grade
The chemotherapy induced anemia market share is segmented into grade 1, grade 2, and grade 3 & 4. The grade 1 segment dominated the global market in 2022 and is expected to remain dominant throughout the forecast period. This is attributed to an increase in prevalence of grade 1 anemia as it is the mildest form characterized by a hemoglobin level of 10-12 g/dL.
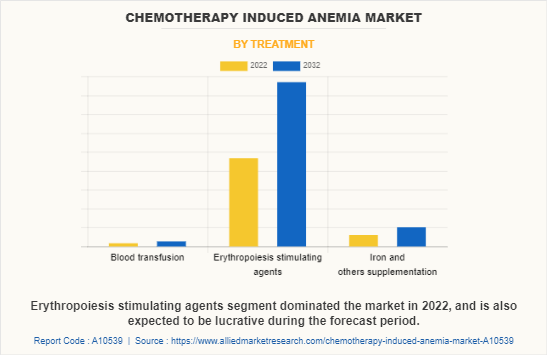
By Treatment
By treatment, the chemotherapy induced anemia market is segmented into blood transfusion, erythropoietin-stimulating agents, iron, and other supplements. The erythropoietin-stimulating agents (ESAs) segment dominated the global market in 2022 and is expected to remain dominant throughout the forecast period, owing to rise in number of patients seeking ESAs as they are effective in treating anemia in cancer patients. In addition, ESAs are convenient to administer as compared to other treatments such as blood transfusion.
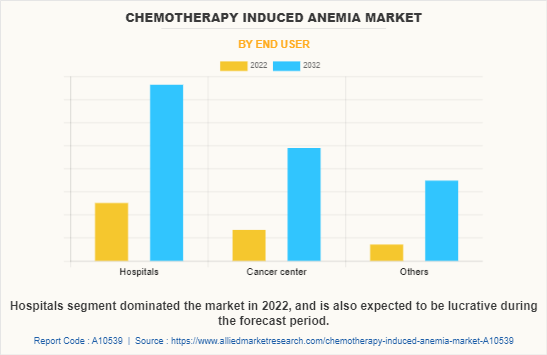
By End User
By end user, the chemotherapy induced anemia market is segregated into hospitals, cancer centers, and others. The hospital segment dominated the global market in 2022 and is anticipated to continue this trend during the forecast period. This is attributed to rise in incidences of cancer and high prevalence of anemia in patients receiving inpatient chemotherapy treatment.
By Region
The chemotherapy induced anemia market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major share of the chemotherapy induced anemia market in 2022 and is expected to maintain its dominance during the forecast period.
The presence of several chemotherapy induced anemia industry, such as Pfizer Inc., Novartis AG, F. Hoffmann La-Roche Ltd., and Astellas Pharma and the rise in the prevalence of cancer in the region drive the growth of the market. For instance, according to National Cancer Institute (NCI), in 2020, an estimated 1,806,590 new cases of cancer will be diagnosed in the U.S. In addition, strong presence of various cancer research institutes such as American Association for Cancer Research (AACR), Mayo Clinic Cancer Center, and National Cancer Institute (NCI) and strong research infrastructure, with many research funding agencies has led to the development of a robust research ecosystem that supports the growth of the chemotherapy induced anemia market. Furthermore, the increase in demand for chemotherapy induced anemia treatment due to the increase in appearance of anemia further contributes toward the market growth. Moreover, easy accessibility of erythropoietin-stimulating agents, iron and other supplements also boosts the growth of the market.
Asia-Pacific is expected to grow at the highest rate during the forecast period. The market growth in this region is attributed to an increase in prevalence of cancer and a surge in awareness about cancer treatment. Asia-Pacific region has a large population, and the prevalence of cancer is rising in recent years due to ageing population, lifestyle changes, and environmental factors. This has led to an increase in the number of patients undergoing chemotherapy, which in turn increases the demand for anemia treatment. For instance, in 2020, the International Journal of Scientific Research estimated that incidence rate of anemia in breast cancer patients is 62.1% followed by 75% in patients with colorectal cancer. In addition, growing awareness and adoption of chemotherapy induced anemia treatments further propels the market growth. Asia-Pacific offers profitable opportunities for key players operating in the chemotherapy induced anemia market, thereby registering the fastest growth rate during the forecast period, owing to the rise in disposable income as well as increase in healthcare expenditure.
Competition Analysis
Competitive analysis and profiles of the major players in the chemotherapy induced anemia market include Pfizer Inc., Dr. Reddy’s Laboratories Ltd., F. Hoffmann-La Roche Ltd., Johnson & Johnson, Amgen Inc., Novartis AG, 3SBio Group, Bristol-Myers Squibb Company, FibroGen, Inc., and Astella Pharma. Major players have adopted partnership as a key developmental strategy to improve their product portfolio and gain a strong foothold in the chemotherapy induced anemia market.
Recent Partnership in the Chemotherapy Induced Anemia Market
In March 2022, Cipla Medpro a leading pharmaceutical subsidiary of Cipla Limited partnered with mAbxience, a biosimilar development company in Argentina, to provide affordable oncology biosimilars to South Africa. Under this partnership, Cipla will market and distribute mAbxience's oncology biosimilars in South Africa. This will allow cancer patients in South Africa to access affordable and effective treatments for chemotherapy-induced anemia and other oncology-related conditions.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the chemotherapy induced anemia market analysis from 2022 to 2032 to identify the prevailing chemotherapy induced anemia market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the chemotherapy induced anemia market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global chemotherapy induced anemia market trends, key players, market segments, application areas, and market growth strategies.
Chemotherapy Induced Anemia Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 5 billion |
| Growth Rate | CAGR of 6.3% |
| Forecast period | 2022 - 2032 |
| Report Pages | 249 |
| By Grade |
|
| By Treatment |
|
| By End User |
|
| By Region |
|
| Key Market Players | Pfizer Inc., Novartis AG, FibroGen, Inc., F. Hoffmann-La Roche Ltd., Astellas Pharma Inc., Bristol-Myers Squibb Company, Johnson & Johnson, 3SBio Group, Dr. Reddy’s Laboratories Ltd., Amgen Inc. |
Analyst Review
Chemotherapy-induced anemia (CIA) is a common side effect of cancer treatment. It occurs when chemotherapy drugs suppress the production of red blood cells in the bone marrow, leading to low levels of hemoglobin in the blood. Development of novel treatment options and increase in R&D activities for the development of new treatment options with minimum side are the key factors that boost the growth of the market.Furthermore, the surge in the development of a new treatment by the key players is expected to create opportunities for the market growth.
For instance, in January 2023, Bristol Myers Squibb Company, a global pharmaceutical company received supplemental biologics license application for Reblozyl (luspatercept-aamt) as a first-line treatment for anemia in adults with lower-risk myelodysplastic syndromes (MDS) from the U.S. Food and Drug Administration (FDA). In addition, the European Medicines Agency (EMA) has validated the application for Reblozyl for the same indication. If approved, Reblozyl can become a new treatment option for patients with lower-risk MDS who experience anemia.
The top companies that hold the market share in chemotherapy induced anemia market are Pfizer Inc., Dr. Reddy’s Laboratories Ltd., F. Hoffmann-La Roche Ltd., Johnson & Johnson, Amgen Inc., Novartis AG, 3SBio Group, Bristol-Myers Squibb Company, FibroGen, Inc., and Astella Pharma.
Asia-Pacific is anticipated to witness lucrative growth during the forecast period, owing to rise in incidence of cancer, surge in healthcare expenditure, and increase in awareness of cancer and its treatment options
The key trends in the chemotherapy induced anemia market are due to Increase in prevalence of cancer, and rise in demand for chemotherapy induced anemia
The base year for the report is 2022.
North America is the largest regional market for chemotherapy induced anemia Market
The total market value of chemotherapy induced anemia Market market is $2,709.6 million in 2022 .
The forecast period in the report is from 2023 to 2032
Major restraints in the chemotherapy induced anemia market are side effects associated with erythropoiesis stimulating agents
Loading Table Of Content...
Loading Research Methodology...



