Cystatin C Assay Market Research, 2033
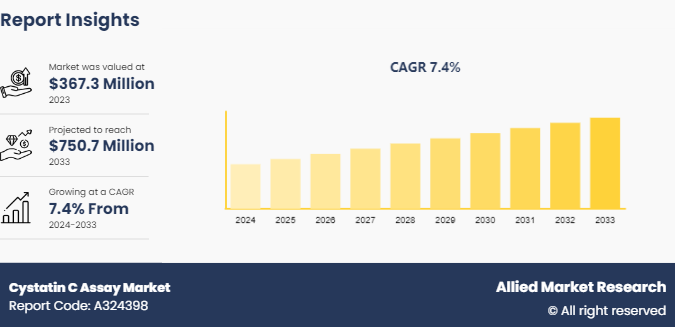
The global cystatin C assay market size was valued at $367.3 million in 2023, and is projected to reach $750.7 million by 2033, growing at a CAGR of 7.4% from 2024 to 2033. The growing prevalence of chronic kidney diseases, the need for accurate and early diagnosis of renal function, and advancements in assay technologies that enhance diagnostic precision and reliability are the major factors which drives the market growth.
Market Introduction and Definition
A cystatin C assay is a diagnostic test that measures the level of cystatin C, a protein produced by all nucleated cells in the blood. Cystatin C serves as a biomarker for kidney function, offering a reliable indicator of glomerular filtration rate (GFR) . Unlike creatinine, cystatin C levels are less influenced by factors such as age, sex, and muscle mass, making it a more precise marker for renal health. Elevated cystatin C levels indicate impaired kidney function and are associated with increased risks of cardiovascular events and mortality. The assay is commonly used to assess and monitor kidney disease progression.
Key Takeaways
The cystatin C assay market share study covers 20 countries. The research includes a segment analysis of each country in terms of value for the projected period.
More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major cystatin C assay industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
The increasing prevalence of CKD globally, attributed to aging population, rising incidences of diabetes and hypertension, and lifestyle changes contributing to renal complications, drives the growing demand for accurate diagnostic tools like the cystatin C assay. In addition, rise in advancements in assay technology, such as automated platforms and high-sensitivity assays that offer rapid results with minimal sample volumes, have enhanced diagnostic accuracy and efficiency, further boost growth duting the cystatin C assay market forecast period. Furthermore, regulatory support for biomarker validation and standardization, coupled with growing investments in healthcare infrastructure in emerging economies, also supports the cystatin C assay market growth.
However, stringent quality control measures and standardized protocols for assay validation poses regulatory and operational challenges, potentially hindering the market expansion. In addition, the limited awareness and training among healthcare professionals regarding the clinical utility and interpretation of Cystatin C results presents barriers to market growth.
Furthermore, advancements in assay technologies, such as point-of-care testing devices and multiplex assay platforms, present opportunities to enhance accessibility and efficiency in clinical settings. In addition, strategic collaborations between healthcare providers, diagnostic laboratories, and assay manufacturers to expand market reach and educate healthcare professionals provides a cystatin C assay market opportunity. Moreover, innovations in high-sensitivity assays and automated platforms enable rapid and precise measurement of Cystatin C levels from smaller sample volumes, enhancing diagnostic efficiency and reducing turnaround times in clinical laboratories further supporting the market growth.
Prevalence Statistics for Chronic Kidney Diseases
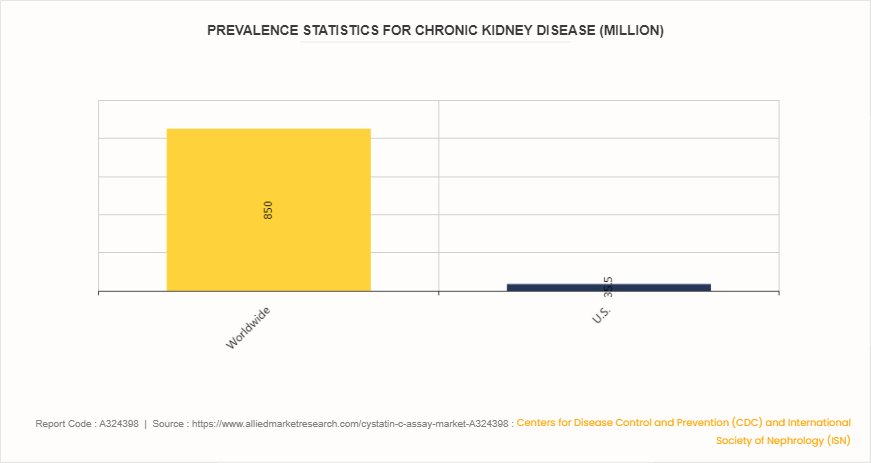
According to the Centers for Disease Control and Prevention (CDC) and the International Society of Nephrology (ISN) , in 2023, the chronic kidney disease (CKD) affected approximately 850 million people worldwide, with about 35.5 million cases in the U.S. alone. This high prevalence drives the critical need for accurate and early diagnosis of CKD, driving the demand for reliable diagnostic tools like the cystatin C assay. Cystatin C is a key biomarker for assessing kidney function, offering advantages over traditional creatinine measurements. Its levels are less influenced by external factors such as age, sex, and muscle mass, providing a more precise estimation of glomerular filtration rate (eGFR) . In addition, the rising global prevalence of CKD necessitates improved diagnostic practices, positioning cystatin C assays as essential tools in clinical settings. As healthcare systems prioritize early detection and management of CKD, the cystatin C assay market size is expected to expand significantly.
Market Segmentation
The cystatin C assay market is segmented into product, method, sample type, application, end user, and region. On the basis of the product, the market is segmented into analyzers and reagents and kits. As per method, the market is classified into enzyme linked immunosorbent assay, particle enhanced turbidimetric immunoassay, particle enhanced nephelometric immunoassay, chemiluminescent immunoassay, immunofluorescence assay, and others. By sample type, the market is bifurcated into blood and urine. By application, the market is classified into diagnostics and research. By end user, the market is classified into hospitals, pharmaceutical and biotechnology companies, and others. Region wise, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional/Country Market Outlook
North America plays a pivotal role in cystatin C assay market share, owing to advanced healthcare infrastructure and high adoption rates of innovative diagnostic technologies which facilitate widespread use of cystatin C assays. In addition, strong support from regulatory bodies, incorporation of cystatin C testing in clinical guidelines, and increase in awareness among healthcare professionals and patients about the importance of early CKD detection further drives the market growth in this region.
According to American Medical Association, the health spending in the U.S. increased by 4.1% in 2022 ($4.4 trillion) . This significant rise in healthcare expenditure drives the cystatin C assay market growth by enabling greater investment in advanced diagnostic tools and technologies.
Asia-Pacific is witnessing rapid growth driven by increasing healthcare expenditure which enable better access to advanced diagnostic technologies. In addition, government initiatives and policies aimed at improving healthcare quality and early disease detection bolster market adoption. Further, rising awareness among healthcare providers and patients about the importance of early CKD diagnosis contributes to market growth.
Industry Trends
As per an article published by National Center for Biotechnology and Information (NCBI) in 2023, The Kidney Disease Improving Global Outcomes (KDIGO) guidelines endorse the use of cystatin C or a clearance measurement for confirmatory testing of estimated glomerular filtration rate (eGFR) from creatinine. For adults with eGFRcreat 45–59 mL/min/1.73 m2 who do not have markers of kidney damage, measuring cystatin C is suggested to confirm the diagnosis of CKD. By recommending cystatin C for confirmatory testing of eGFR, particularly in adults with specific eGFRcreat ranges without other kidney damage markers, these guidelines highlight the clinical importance of cystatin C in diagnosing chronic kidney disease (CKD) thereby supporting the market growth.
In March 2024, Gentian Diagnostics, a leading manufacturer of cystatin C, announced its commitment to kidney care following the release of the KDIGO 2024 Clinical Practice Guideline for Chronic Kidney Disease (CKD) . The guideline emphasizes the pivotal role of cystatin C in estimating glomerular filtration rate (GFR) and tailoring treatment decisions based on individual kidney function status, especially when combined with creatinine. The latest guidelines, recommended for increased use of cystatin C, aim to improve patient care thereby supporting market growth.
Competitive Landscape
The major players operating in the cystatin C assay market include Abbott, F. Hoffmann-La Roche Ltd, Siemens, Thermo Fisher Scientific Inc., Randox Laboratories Ltd., DiaSys Diagnostic Systems GmbH, Agilent Technologies, Inc., Abcam Limited, Sino Biological, Inc., and Gentian Diagnostics ASA. Other players in cystatin C assay market include Getein Biotech, Inc., Eurolyser Diagnostica GmbH and so on.
Recent Key Strategies and Development in Cystatin C Assay Industry
In September 2022, ?Gentian Diagnostics ASA announced the Gentian Cystatin C and GCAL assays received IVDR certified by TüV.
Key Sources Referred
- National Center for Biotechnology and Information (NCBI)
- Centers for Medicare & Medicaid Services (CMS)
- National Health Service (NHS)
- Australian Government Department of Health and Aged Care
- Government of Canada's Health and Wellness
- Ministry of Health and Family Welfare (MoHFW)
- National Health Mission (NHM)
- Ayushman Bharat - Health and Wellness Centres (AB-HWCs)
- Centers for Disease Control and Prevention (CDC)
- Food and Drug Administration (FDA)
- National Institutes of Health (NIH)
- World Health Organization (WHO)
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the cystatin c assay market analysis from 2024 to 2033 to identify the prevailing cystatin c assay market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the cystatin c assay market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global cystatin c assay market trends, key players, market segments, application areas, and market growth strategies.
Cystatin C Assay Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 750.7 Million |
| Growth Rate | CAGR of 7.4% |
| Forecast period | 2024 - 2033 |
| Report Pages | 233 |
| By Product |
|
| By Method |
|
| By Sample Type |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | DiaSys Diagnostic Systems GmBH, Thermo Fisher Scientific Inc., Abbott, Agilent Technologies, Inc., Siemens, F. Hoffmann-La Roche Ltd , Abcam Limited, Sino Biological, Inc., Gentian Diagnostics ASA, Randox Laboratories Ltd. |
The forecast period for cystatin C assay market is 2024 to 2033.
The market value of cystatin C assay market in 2033 is $750.7 million.
The total market value of cystatin C assay market is $367.3 million in 2023.
The market growth is driven by the increasing prevalence of chronic kidney diseases, advancements in diagnostic technologies, and the need for accurate and early diagnosis of renal function.
The base year is 2023 in cystatin C assay market.
A cystatin C assay is a diagnostic test that measures the level of cystatin C in the blood, a protein that serves as a biomarker for kidney function. It is used to assess glomerular filtration rate (GFR) and diagnose renal conditions.
Loading Table Of Content...



