Embolization Particle Market Research, 2033
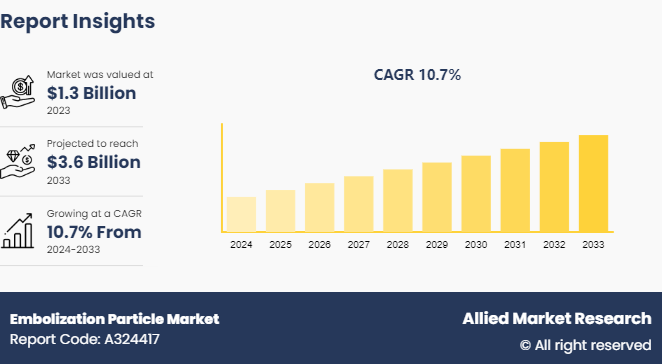
The global embolization particle market size was valued at $1.3 billion in 2023, and is projected to reach $3.6 billion by 2033, growing at a CAGR of 10.7% from 2024 to 2033. The embolization particles market is driven by the increasing prevalence of cancer and vascular diseases, technological advancements in embolization techniques, and the growing demand for minimally invasive procedures. Additionally, rising healthcare expenditures and supportive government policies further bolster market growth.
Market Introduction and Definition
Embolization particles are specialized medical devices used in a minimally invasive procedure known as embolization. This technique involves the intentional occlusion or blockage of blood vessels to restrict blood flow to specific areas of the body. The particles are typically small, ranging from micrometers to millimeters in size, and can be made from various materials such as polyvinyl alcohol (PVA) tris-acryl gelatin microspheres, or biodegradable substances.
The primary goal of embolization is to treat a variety of medical conditions, including tumors, arteriovenous malformations, and uterine fibroids, by depriving them of their blood supply. This can lead to the shrinkage or destruction of abnormal tissues. Embolization particles are carefully selected based on their size, shape, and composition to ensure precise targeting and effective blockage of the intended blood vessels, minimizing the risk to surrounding healthy tissues. This procedure is often guided by imaging techniques like angiography, ensuring accurate delivery of the particles and optimal therapeutic outcomes.
Key Takeaways
- The embolization particle market study covers 20 countries. The research includes a segment analysis of each country in terms of value ($Billion) for the projected period 2023-2033.
- More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major embolization particle industry participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
The embolization particle market size is driven by several key factors, including the increasing prevalence of chronic diseases such as cancer and vascular disorders, which necessitate effective and minimally invasive treatment options. Technological advancements in the development of embolization particles, such as the introduction of drug-eluting beads and biodegradable materials, enhance the efficacy and safety of procedures, thereby boosting market growth. Additionally, the growing adoption of minimally invasive surgical techniques, owing to their benefits like reduced recovery times and lower complication rates, is driving demand for embolization particles. For instance, the use of embolization in treating liver cancer and uterine fibroids has become more prevalent due to these advantages.
However, the market faces certain restraints during embolization particle market forecast, such as the high cost of embolization procedures and the associated medical devices, which can limit their accessibility, particularly in developing regions. Additionally, the requirement for skilled healthcare professionals to perform embolization procedures can pose a challenge in areas with a shortage of trained personnel. Regulatory hurdles and the lengthy approval processes for new embolization products can also hinder market growth, as they may delay the introduction of innovative treatments to the market.
Despite these challenges, the embolization particle market offers significant embolization particle market opportunity for growth. The expanding applications of embolization techniques, such as in the treatment of gastrointestinal bleeding and prostate artery embolization for benign prostatic hyperplasia (BPH) , present new avenues for market expansion. Moreover, the improving healthcare infrastructure in emerging markets, coupled with increasing healthcare expenditure, is expected to enhance access to advanced medical procedures, including embolization. Strategic collaborations and acquisitions among key market players are fostering innovation and expanding the reach of embolization treatments. For example, partnerships between medical device companies and research institutions can lead to the development of cutting-edge embolization technologies, further driving market growth.
Market Segmentation
The embolization particle industry is segmented into particle type, application, end user and region. On the basis of particle type, the market is classified into embolization particle, microspheres, particles, drug eluting beads, PVA particles and gelfoam particles, and others. Based on application, the market is divided into oncology, peripheral vascular disease, neurovascular disease, urology, and others. As per end user, the market is segmented into hospitals, ambulatory surgical centers, and others. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional/Country Market Outlook
North America has largest embolization particle market share and is primarily driven by several key factors such as increasing prevalence of cancer, particularly liver and renal cancers, which necessitate embolization procedures as a crucial treatment option. The region's advanced healthcare infrastructure supports the adoption of innovative medical technologies, making it a favorable environment for the use of sophisticated embolization techniques. Additionally, the growing preference for minimally invasive procedures, which offer reduced recovery times and lower risks compared to traditional surgeries, significantly boosts market demand.
The aging population in North America also contributes to the market's growth, as older individuals are more prone to conditions such as vascular diseases and tumors that require embolization. Furthermore, strong investment in healthcare by both public and private sectors, alongside the presence of major market players driving continuous product development and innovation, propels the embolization particle market growth. Regulatory support and favorable reimbursement policies further enhance the accessibility and adoption of embolization procedures in the region.
Industry Trends
- Innovations in embolization particle materials and delivery systems are enhancing the precision and efficacy of embolization procedures. Developments in biodegradable particles and drug-eluting beads are particularly noteworthy, offering targeted and controlled therapeutic effects.
- There is a global shift towards minimally invasive surgical techniques due to their benefits, such as reduced recovery times, lower risk of complications, and decreased hospital stays. Embolization, being a minimally invasive procedure, is gaining popularity among healthcare providers and patients alike.
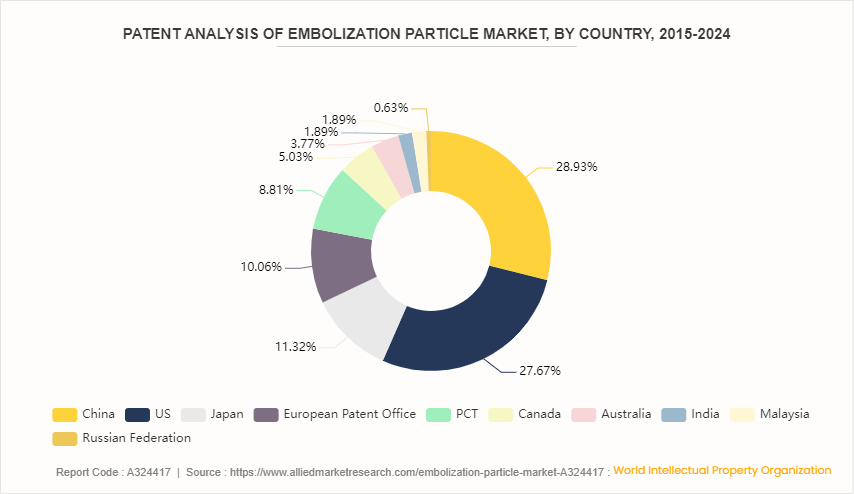
Patent Analysis, By Country, 2015-2024
China witnessed the highest number of patent approvals and applications, due to favorable government policies, new technological advancement, and new product launches in the country. U.S. has 27.67% of the total number of patents, followed by Japan at 11.32% and European Patent Office at 10.06%.
Competitive Landscape
The major embolization particle market share players operating in the embolization particle market include Boston Scientific Corporation, Medtronic plc., Merit Medical Systems, Inc., Terumo Corporation, Cook Medical, Stryker Corporation, Penumbra, Inc., Sirtex Medical Limited, Johnson & Johnson and Kaneka Corporation. Other players in Embolization particle market include Guerbet, Shape Memory Medical Inc., Terumo Group Company, and so on.
Recent Key Strategies and Developments
- In June 2023, Terumo Aortic's worldwide Post-Approval Study (PAS) , codenamed EXTEND, is focused on Thoraflex Hybrid, the only Frozen Elephant Trunk (FET) device approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with complex aortic arch sickness.
- In May 2023, Merit Medical Systems, Inc. acquired the BioSentry® Biopsy Tract Sealant System and various dialysis catheter devices from AngioDynamics, Inc. for a total cash payment of $100 million. Merit also stated that it recently paid $32.5 million in cash to Bluegrass Vascular Technologies, Inc. for the Surfacer Inside-Out Access Catheter System.
- In December 2022, Sirtex Medical and Grand Pharmaceutical Group Limited have succeeded in getting their SIR-Spheres Y-90 resin microspheres approved by the Chinese National Medical Products Administration. The first-ever effective selective internal radiation therapy (SIRT) surgery was carried out utilizing SIR-Spheres in China.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the embolization particle market segments, current trends, estimations, and dynamics of the embolization particle market analysis from 2023 to 2033 to identify the prevailing embolization particle market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the embolization particle market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global embolization particle market statistics.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global embolization particle market trends, key players, market segments, application areas, and market growth strategies.
Key Sources Referred
- Centers for Disease Control and Prevention
- World Health Organization
- National Center for Biotechnology Information
- The Lancet
- The Scottish Government
- Science Direct
- Health Resources and Services Administration (HRSA)
- Department of Health and Human Services (HHS)
- National Institutes of Health (NIH)
- GOV.UK
Embolization Particle Market , by Type Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 3.6 Billion |
| Growth Rate | CAGR of 10.7% |
| Forecast period | 2024 - 2033 |
| Report Pages | 260 |
| By Type |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | Stryker Corporation., Penumbra, Inc., Terumo Corporation, Sirtex Medical Limited, Cook Medical, Medtronic plc, Kaneka Corporation., Johnson & Johnson, Merit Medical Systems, Inc., Boston Scientific Corporation |
The Boston Scientific Corporation, Medtronic plc., Merit Medical Systems, Inc., Terumo Corporation held a high market position in 2023.
The base year is 2023 in Embolization Particle market.
The forecast period for Embolization Particle market is 2024 to 2033.
The market value of Embolization Particle market is projected to reach $3.6 million by 2033.
The total market value of Embolization Particle market was $1.3 billion in 2023.
Loading Table Of Content...



