Epilepsy Drugs Market Research, 2032
The global epilepsy drugs market size was valued at $7 billion in 2022, and is projected to reach $9.8 billion by 2032, growing at a CAGR of 3.5% from 2023 to 2032. Epilepsy is a neurological disorder that causes recurring seizures due to abnormal electrical activity in the brain. It is a chronic noncommunicable disease. There are many different types of seizures, but all involve involuntary movements that may affect a part of the body or the entire body. Treatment options include medication such as first, second and third generation drugs. The exact cause of epilepsy is often unknown, but it can be attributed to various factors, including genetic, brain injury, infections, and prenatal factors. Seizures occur in epilepsy classified into focal seizures, generalized seizures, and non-epileptic seizures. Treatment for epilepsy usually involves antiepileptic medications which help to control seizures. The choice of medication and dosage depends on the type of seizures, age of the patient, overall health, and other factors.
Market Dynamics
Rise in number of people affected with epilepsy has increased the demand for epilepsy drugs, which drives the epilepsy drugs market growth. Epilepsy is a common neurological disorder, and the surge in prevalence of epilepsy worldwide is a significant driving factor for the epilepsy drugs market. In addition, the burden of epilepsy has increased owing to factors such as population growth, aging population, and improved diagnosis which support the market growth. For instance, according to an article published by UpToDate Inc., in August 2021, the incidence rate of epilepsy increases with age and is highest in patients above 75 years of age. Thus, rise in prevalence of epilepsy among geriatric population drives the demand for epilepsy drugs, thus boosting the market growth.
Furthermore, there is a rise in geriatric population as they are more to prone to various age-related neurological disorders, including epilepsy which drive the market growth. As the global population continues to age, the incidence of epilepsy in the elderly is expected to increase, which drives the demand for epilepsy medications. Older adults may require specific treatment considerations due to age-related factors, such as comorbidities and polypharmacy, creating a need for specialized medications and management approaches. Thus, rise in geriatric population drives the demand for epilepsy drugs and further supports the market growth. Furthermore, there has been ongoing R&D in the field of epilepsy drugs, leading to the discovery of new antiepileptic drugs (AEDs) with improved efficacy, safety, and tolerability profiles. These advancements in drug development, including the introduction of generic versions of antiepileptic drugs, have expanded the options for treating epilepsy and driving the growth of the epilepsy drugs market.
Certain lifestyle changes and risk factors, such as stress, lack of sleep, alcohol & drug abuse, and head injuries, can trigger or worsen seizures in people with epilepsy. As these risk factors become more prevalent in modern lifestyles, the demand for epilepsy drugs is expected to increase, and further drive the market growth. Moreover, rise in healthcare expenditure and favorable reimbursement policies for epilepsy drugs in some regions are also augment the epilepsy drugs market growth. Improved access to healthcare services and reimbursement support for epilepsy medications can increase the affordability and utilization of epilepsy drugs, thus contributing to market growth.
Online pharmacy has become a vital tool for small and large businesses globally, due to rise in preference of consumers for online shopping over traditional purchasing methods this has supports the market growth. The demand for epilepsy drug is not only limited to developed countries but is also being witnessed in the developing countries, such as China, Brazil, and India, which fuel the growth of the epilepsy drugs market size.
In some regions, reimbursement policies for epilepsy drugs may be limited or inadequate, resulting in higher out-of-pocket costs for patients. This can impact the affordability and utilization of epilepsy drugs, particularly for patients with limited financial resources. In addition, limited access to healthcare services, particularly in rural or underserved areas, can hinder the diagnosis and treatment of epilepsy, leading to lower demand for epilepsy drugs. Adverse effects of epilepsy drugs such as cognitive impairment, behavioral changes, and drug interactions can limit the use of certain epilepsy drugs and affect patient adherence to treatment led to restrain the market growth.
The outbreak of COVID-19 disrupted workflows in the health care sector around the world. The pandemic forced a number of industries to shut their doors temporarily, including several sub-domains of health care. The global epilepsy drugs industry experienced a decline in 2020 due to global economic recession led by COVID-19. In addition, the COVID-19 outbreak disrupted the supply chain across various end-user industries such as pharmaceutical and medical devices companies. In addition, decrease in sales of epilepsy drugs during pandemic further restrain the market growth.
However, the market is recovering after pandemic, and show stable growth for epilepsy drugs market. This is attributed to the increase in adoption antiepileptic drugs, rise in cases of epilepsy, and rise in development of new epilepsy drugs.
Epilepsy Drugs Market Segmental Overview
The epilepsy drugs market is segmented into seizure type, drug generation, distribution channel and region. By seizure type, the market is categorized into focal seizures, generalized seizures, and non-epileptic seizures. On the basis of drug generation, the market is segregated into first generation drugs, second generation drugs, and third generation drugs. By distribution channel, the market is classified into hospital pharmacies, drug stores and retail pharmacies, and online providers. Region wise, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
By Seizure Type Segment Review
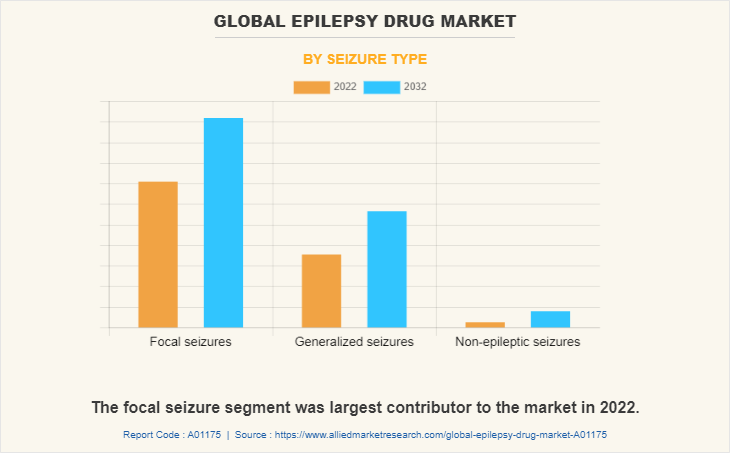
The focal seizures segment dominated the global market in 2022 and is expected to remain dominant throughout the forecast period. As focal seizures are highly prevalent, the demand for effective medications to manage focal seizures is significant, which can drive the segment growth.
By Drug Generation Segment Review
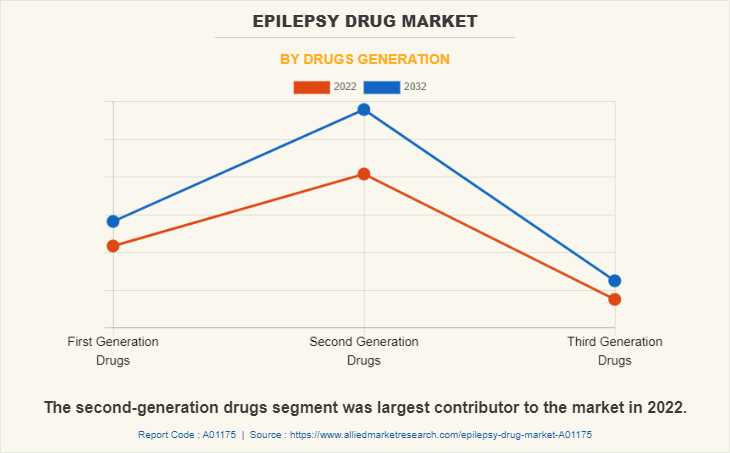
The second-generation drugs segment dominated the global epilepsy drugs market share in 2022 and is anticipated to continue this trend during the forecast period. This was attributed to high adoption of second-generation drugs as they show improved efficacy in epilepsy patients, high safety profile, and novel mechanisms of action.
However, third generation drugs segment is expected to register a highest CAGR during the forecast period owing to rise in adoption of third generation drugs as they have high effectiveness in controlling seizures, including drug-resistant seizures, and used in patients with epilepsy who may not have responded adequately to first- or second-generation drugs.
By Distribution Channel Segment Review
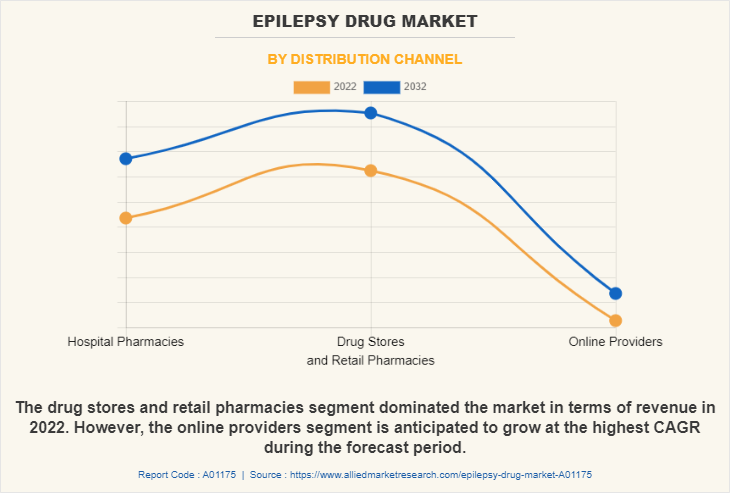
The drug stores and retail pharmacies held the largest market epilepsy drugs market share in 2022 and is expected to remain dominant throughout the forecast period, owing to easy and rapid access to a broad range of drugs and adequate products in stock offered by these distribution channel, and availability of these products at retail stores at discounted price. However, the online providers segment is expected to register the highest CAGR during the forecast period. This is attributed to easy accessibility and availability of epilepsy drugs and rise in technological advancements for online platform.
By Region Segment Review
The epilepsy drugs market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major share of the epilepsy drugs market in 2022 and is expected to maintain its dominance during the forecast period. The presence of several major players, such as Novartis AG, and Abbott Laboratories, and high adoption of epilepsy drugs in this region drive the growth of the market.
Furthermore, high healthcare expenditure, high purchasing power, and rise in adoption rate of epilepsy drugs are expected to drive the market growth. Moreover, product launch, and product approvals adopted by the key players in this region boost the growth of the market.
Asia-Pacific is expected to grow at the highest rate during the forecast period. The market growth in this region is attributable to presence of pharmaceutical companies in the region as well as growth in the purchasing power of populated countries, such as China and India. Moreover, the rise in healthcare expenditure and rise in awareness about early diagnosis and treatment drive the growth of the market in this region. Furthermore, the Asia-Pacific region exhibits the largest medicine supply and the largest pharmaceuticals industry with availability of raw material in abundance, which can be easily accessed by manufacturers of epilepsy drug. This, in turn, drives the growth during epilepsy drugs market forecast.
Competition Analysis
Competitive analysis and profiles of the major players in the epilepsy drug, such as Novartis AG, GlaxoSmithKline plc, Sanofi, UCB S.A., Abbott Laboratories, Bausch Health Companies, Inc, Viatris Inc., Johnson & Johnson, Sumitomo Pharma Co., Ltd, and H. Lundbeck A/S. Major players have adopted product approval as key developmental strategy to improve the product portfolio of the epilepsy drugs market.
Recent Product Approval in the Epilepsy Drugs Market
In November 2020, UCB S.A., a global pharmaceutical company announced that the U.S. Food and Drug Administration (FDA) has approved VIMPAT (lacosamide) CV as adjunctive therapy in the treatment of primary generalized tonic-clonic seizures (PGTCS) in patients four years of age and older and VIMPAT injection for intravenous use in children four years of age and older.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the epilepsy drugs market analysis from 2022 to 2032 to identify the prevailing epilepsy drugs market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the epilepsy drugs market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global epilepsy drugs market trends, key players, market segments, application areas, and market growth strategies.
Epilepsy Drugs Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 9.8 billion |
| Growth Rate | CAGR of 3.5% |
| Forecast period | 2022 - 2032 |
| Report Pages | 290 |
| By Seizure Type |
|
| By Drugs Generation |
|
| By Distribution Channel |
|
| By Region |
|
| Key Market Players | Sanofi, GlaxoSmithKline plc, Sumitomo Pharma Co., Ltd, Abbott Laboratories, Novartis AG, Viatris Inc., H. Lundbeck A/S, UCB S.A., Johnson & Johnson, Bausch Health Companies, Inc. |
Analyst Review
The increase in demand for epilepsy drugs and rise in awareness about importance of early diagnosis and treatment is expected to offer profitable opportunities for the expansion of the market. However, side effects of epilepsy drugs such as drowsiness, fatigue, and gastrointestinal symptoms hinder the market growth.
The surge in incidences of epilepsy drives the demand for antiepileptic drugs and supports the market growth. Moreover, rise in awareness about increase in number of generic drug manufactures and increase in ageing population, as they are more prone to develop epilepsy, are some factors that further boost market growth.
Furthermore, North America is expected to witness highest growth, in terms of revenue, owing to the rise in prevalence of epilepsy along with surge in geriatric population and presence of major key players in the region. However, Asia-Pacific is anticipated to witness notable growth owing to rise in healthcare awareness and increase in incidences of epilepsy.?
Epilepsy drugs, also known as antiepileptic drugs (AEDs) or antiseizure medications, are a class of medications used to manage and treat epilepsy.
Rise in prevalence of epilepsy condition, increase in awareness about epilepsy and its treatment options among the general population and healthcare professionals, and significant progress in the development of newer and more effective epilepsy drugs are the factors responsible for the market growth.
Epilepsy is a neurological disorder characterized by recurring seizures. Seizures occur when there is abnormal electrical activity in the brain, which can cause a variety of symptoms depending on the type and severity of the seizure
The estimated industry size of epilepsy drugs in 2032 is $9,802.45 million.
Top companies such as Novartis AG, Sanofi, Abbott Laboratories, GlaxoSmithKline plc and Johnson & Johnson held high market share in 2022.
The forcast period for epilepsy drugs market is 2023 to 2032
North America is the largest regional market for epilepsy drugs owing to high prevalence of epilepsy, favorable government initiatives, growing awareness about epilepsy, and the presence of major pharmaceutical companies which led to the availability of a wide range of epilepsy drugs in the region.
Second generation drugs segment dominated the global market in 2022, owing to high adoption of second generation drugs due to their improved efficacy and higher safety profiles compared to first-generation drugs.
The base year is 2022 in epilepsy drugs market.
Loading Table Of Content...
Loading Research Methodology...



