Major Advantages
-
The report under production by Allied Market Research on the Hypoparathyroidism global clinical trials market offers a comprehensive study of global market size & forecast, country-level outlook, segmental analysis, market opportunities, major determinant factors of the growth, and key trends.
-
Porter’s five forces model showcases the effectiveness of buyers & sellers, which is vital to aid the market players to adopt fruitful strategies. Moreover, the report includes
-
Threat of new entrants
-
Threat of substitutes
-
Bargaining power of suppliers
-
Bargaining power of consumers
-
Competition among key players
-
-
A detailed analysis of the driving and restraining factors of the global Hypoparathyroidism global clinical trials market is offered in the report.
-
The global Hypoparathyroidism global clinical trials market report provides quantitative and qualitative analysis of the market from 2023 to 2032. The qualitative analysis highlights key regulations, value chain analysis, patent analysis, and pain point analysis.
-
Value chain analysis: AMR provides a comprehensive analysis of all the stages coupled with the key stakeholders operating in every stage along with their strategic decisions and impact on the market.
-
Key regulations: AMR offers key regulations and standards for the industries. The section lists some of the regulatory documents of product/service type.
-
Pain points analysis: The report offers insights on challenges faced by key stakeholders currently active in all stages of the value chain along with the strategic decisions taken by other players to maintain their foothold in the market.
-
Competition Analysis
The report presents the analysis of the top companies and analysis of their market share. The report includes company profiles along with comprehensive information regarding market share, company description, key developments, and financial details. In addition, the company profiles section contains the data about the company’s product/services and brand names. Key players identified in this report are AstraZeneca, Merck and Co., Inc., Novartis AG, Pfizer Inc., AbbVie Inc., McKesson Corporation, Eli Lilly and Company, F. Hoffmann-La Roche Ltd., Teva Pharmaceutical Industries Ltd., GlaxoSmithKline plc.
Hypoparathyroidism global clinical trials market Share Analysis, 2023
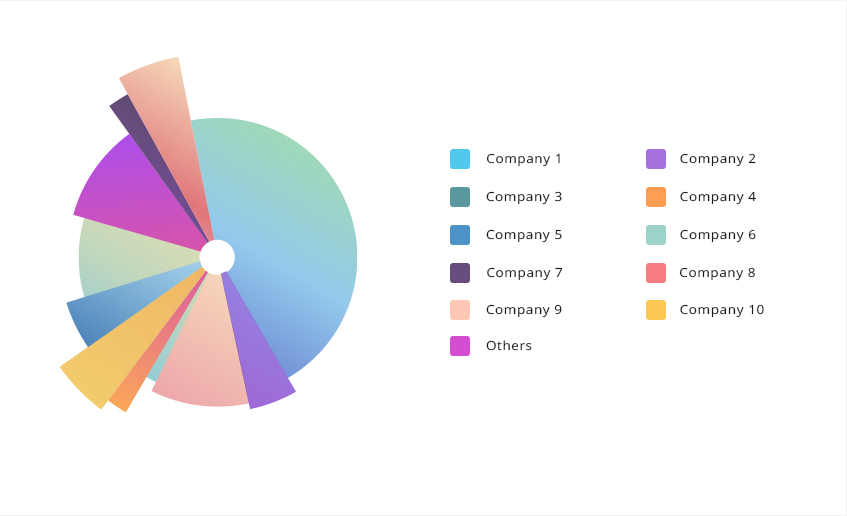
Graph for representation purpose only
Key Classification And Analysis Of The Market
The global Hypoparathyroidism global clinical trials market has been classified into by phase, by type of molecule, by route of administration, by trial status, by trial type, by study type, by sponsors/collaborators. On the basis of region, the market is divided into North America, Europe, Asia-Pacific, and LAMEA. Country wise, the global Hypoparathyroidism global clinical trials market is studied across the U.S., Canada, Mexico, the UK, Germany, France, Spain, Italy, and rest of Europe, China, India, Japan, South Korea, Australia, and the Rest of Asia-Pacific, Latin America, Middle East, and Africa.
The report offers key segmental analysis in both quantitative and qualitative terms, which helps assess the current addressable market and untapped growth opportunities. This analysis is provided for each segment at regional and country levels.
Hypoparathyroidism Clinical Trials Market Report Highlights
| Aspects | Details |
| By Phase |
|
| By Type of Molecule |
|
| By Route of Administration |
|
| By Trial Status |
|
| By Trial Type |
|
| By Study Type |
|
| By Sponsors/Collaborators |
|
| By Region |
|
| Key Market Players | AstraZeneca, Novartis AG, Pfizer Inc., Merck and Co., GlaxoSmithKline plc., F. Hoffmann-La Roche Ltd., McKesson Corporation, AbbVie Inc., Teva Pharmaceutical Industries Ltd., Eli Lilly and Company |
Loading Table Of Content...



