Immune checkpoint inhibitors Market Size & Trends, 2032
Immune checkpoint inhibitors represent a category of drugs utilized in cancer immunotherapy. They function by targeting specific proteins on immune or cancer cells, thereby boosting the body's immune response against cancer. While the immune system serves as a critical defense mechanism against abnormal or cancerous cells, certain mechanisms, known as immune checkpoints, regulate its function, preventing excessive attacks on healthy cells.
Value Projections and Insights
- The global immune checkpoint inhibitors market was valued at $40.1 billion in 2022, and is projected to reach $189.4 billion by 2032, growing at a CAGR of 16.8% from 2023 to 2032.
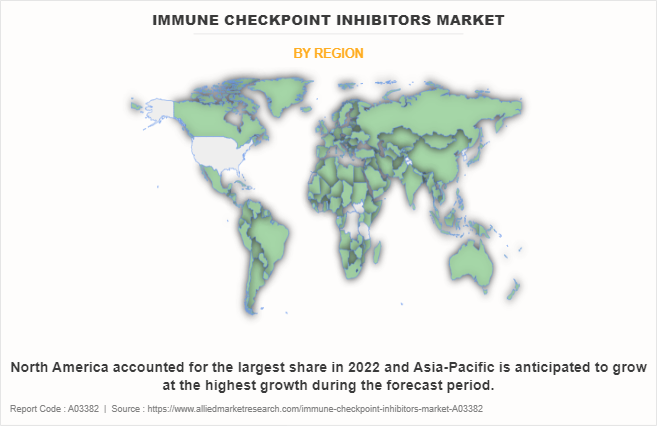
- The Asia-Pacific is expected to register the highest CAGR during the forecast period as prevailing cancer cases and growing development in healthcare infrastructure with rise in adoption and demand for immune checkpoint inhibitors.
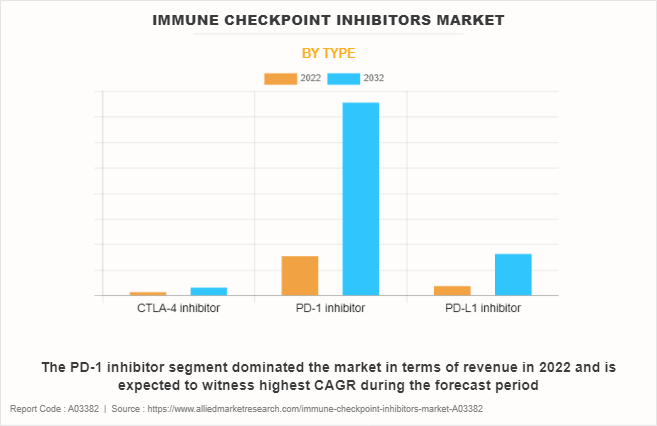
- The PD-1 inhibitor segment holds the largest immune checkpoint inhibitors market share.
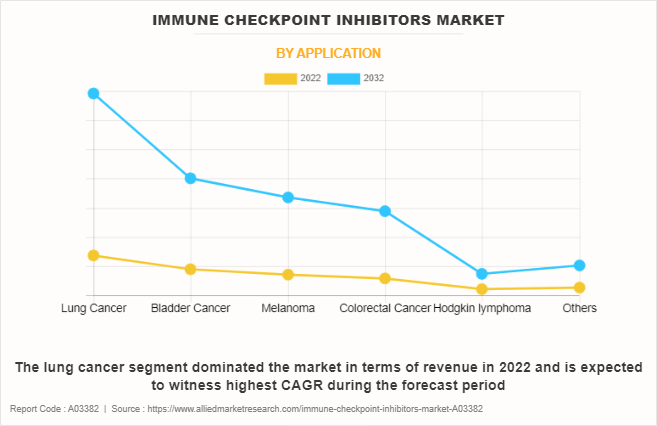
- Lung cancer segment dominated the immune checkpoint inhibitors market share in 2022.

The growing demand for immune checkpoint inhibitors is directly linked to the rising global prevalence of cancer. These inhibitors, such as ipilimumab, pembrolizumab, nivolumab, atezolizumab, avelumab, and durvalumab, are widely used in treating various cancers, including lung, melanoma, bladder, colorectal, and renal cancers. As cancer rates increase globally, so does the need for these therapies. According to Globocan's 2020 estimates, 19.2 million new cancer cases were reported worldwide, with projections suggesting a rise to 26 million by 2030. This surge in cancer cases drives the demand for immune checkpoint inhibitors, making them crucial to the market's ongoing expansion.
However, the high cost of immune checkpoint inhibitors presents a major challenge, limiting accessibility for patients and placing a financial burden on healthcare systems. Despite these barriers, the immune checkpoint inhibitors market shows strong growth potential, particularly in emerging economies. These regions are seeing a rise in cancer incidences due to aging populations, lifestyle changes, and improved diagnostics. As awareness of early detection and treatment options grows, so will the demand for immune checkpoint inhibitors, offering opportunities for market expansion in these untapped areas.
Industry Highlights
- Increasing prevalence of cancers such as lung, melanoma, and colorectal, which fuels demand for these advanced therapies.
- Significant investments in research and development are a major highlight, with pharmaceutical companies and research institutions allocating considerable budgets to develop new inhibitors and improve existing therapies.
- Major players in the industry, including Merck, Bristol-Myers Squibb, and Roche, are scaling up production to meet the growing demand.
- The trend towards combination therapies is gaining traction, as combining immune checkpoint inhibitors market trends with other treatments has shown promising results in clinical trials.
The immune checkpoint inhibitors market is marked by significant growth driven by escalating cancer incidences and advancements in cancer immunotherapy. Consumption is rising as these therapies prove effective across various cancer types, including lung and melanoma. On the production side, key players are expanding their portfolios with innovative inhibitors and combination therapies. Spending in the sector is substantial, reflecting the high cost of these treatments and ongoing investments in research and development. Market dynamics are shaped by increasing demand for effective cancer therapies, competitive pressures from alternative treatments, and the need to address high therapy costs and access issues.
Key Areas Covered in the Report
- Immune checkpoint inhibitors are highly sought-after due to their effectiveness in treating a range of cancers and improving patient outcomes.
- The immune checkpoint inhibitors market faces competition from other cancer treatments such as targeted therapies, chemotherapy, and emerging immunotherapies.
- Ongoing research and development are bringing diversity and new options to the market, with new inhibitors and combination therapies continuously being explored.
- The high cost of these therapies poses challenges, affecting accessibility and leading to ongoing discussions about healthcare spending and insurance coverage.
Immune checkpoint inhibitors are increasingly valued for their effectiveness in treating various cancers and enhancing patient outcomes. This demand is driven by their success in managing previously difficult-to-treat cancers. However, the immune checkpoint inhibitors market is competitive, with other cancer treatments such as targeted therapies, chemotherapy, and emerging immunotherapies posing significant challenges. Ongoing research and development are vital, continuously introducing new inhibitors and combination therapies that diversify treatment options and potentially improve efficacy. Despite these advancements, the high cost of immune checkpoint inhibitors remains a major issue, impacting patient accessibility and sparking debates over healthcare expenditure and insurance coverage. As the market evolves, balancing innovation with affordability will be crucial for expanding access and maximizing the benefits of these therapies.
Topics discussed in the report
- PD-1 inhibitors
- Cancer immunotherapy
- Monoclonal antibodies
- Tumor immune evasion
- CTLA-4 inhibitors
- Combination therapies
Segment Overview
The immune checkpoint inhibitors market is segmented on the basis of product type, application, and region. By product type, the market is categorized into CTLA-4 inhibitor, PD-1 inhibitor, and PD-L1 inhibitor. On the basis of application, the market is classified into lung cancer, bladder cancer, melanoma, colorectal cancer, Hodgkin lymphoma, and others. Region-wise, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, and rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, and rest of LAMEA).
Comparative Matrix of Key Segments
parameters | PD-1/PD-L1 Inhibitors | CTLA-4 Inhibitors |
Market Share | Largest market share, widely used in various cancer types | Moderate, focused on specific cancers |
Target Indications | Lung, melanoma, bladder, colorectal cancers | Melanoma, renal cancer |
Key Players | Merck (Keytruda), Bristol-Myers Squibb (Opdivo), Roche (Tecentriq) | Bristol-Myers Squibb (Yervoy) |
Distribution Channels | Hospitals, oncology clinics, specialty pharmacies | Hospitals, oncology clinics |
Challenges | High cost, competition from other treatments, resistance development | Limited patient response, high toxicity |
Growth Drivers | Expanding indications, favorable clinical outcomes, increasing cancer incidence | Research focus on improving safety and efficacy |
Regulatory Status | Approved for multiple indications worldwide | Approved for specific cancers, ongoing trials |
Regional Dynamics and Competition
The immune checkpoint inhibitors market is characterized by intense regional competition, influenced by varying levels of research investment and differences in economic, legal, and healthcare systems. In North America, particularly the U.S., the market thrives due to substantial research funding and a robust biotechnology infrastructure. Europe, with its active academic and industrial research, remains competitive despite facing stringent regulatory challenges. Meanwhile, the Asia-Pacific region is experiencing rapid growth, driven by increased research investment, a shift towards population medicine, and supportive government policies. This expansion positions Asia-Pacific to potentially surpass other regions in technological advancements. Latin America and the Middle East, though offering opportunities, are progressing at a slower pace due to moderate investment levels and regulatory hurdles. While North America and Europe currently lead, Asia-Pacific’s rapid growth suggests a more diverse and global market landscape in the near future.
Some of the major players analyzed in this report are Merck & Co., Inc., AstraZeneca plc, F. Hoffmann-La Roche Ltd., Merck KGaA, Regeneron Pharmaceuticals, Inc., Bristol-Myers Squibb Company, BeiGene, Ltd., Shanghai Junshi Biosciences Co., Ltd., GlaxoSmithKline plc, and Innovent Biologics, Inc.
Immune Checkpoint Inhibitors Market News Release
- National Cancer Institute, Division of Cancer Control & Population Sciences (DCCPS), stated that, 623,405 people are living with metastatic breast, prostate, lung, colorectal cancer, or metastatic melanoma in the U.S.
- Insurance companies such as Moda Health Plan, Inc., Aetna Inc., Blue Cross Blue Shield Association, Medicare, and Medicaid cover the treatment cost of Yervoy, Keytruda, Opdivo, Tecentriq, Bavencio, and Imfinzi immune checkpoint inhibitors in the U.S.
- According to the Ministry of Health and Family Welfare, India 2022, treatment of cancer under the Pradhan Mantri Jan Arogya Yojana has been one of the prime focus areas to safeguard the beneficiaries from catastrophic expenditure of cancer treatment. Under this scheme, health insurance cover of $0.01 million (Rs. 5 lakhs) per family per year is provided for secondary or tertiary care hospitalization.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the immune checkpoint inhibitors market analysis from 2022 to 2032 to identify the prevailing market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders to make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the immune checkpoint inhibitors market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global immune checkpoint inhibitors market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the immune checkpoint inhibitors market players.
- The report includes the analysis of the regional as well as global immune checkpoint inhibitors market trends, key players, market segments, application areas, and market growth strategies.
Immune Checkpoint Inhibitors Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 189.4 billion |
| Growth Rate | CAGR of 16.8% |
| Forecast period | 2022 - 2032 |
| Report Pages | 248 |
| By Type |
|
| By Application |
|
| By Region |
|
| Key Market Players | AstraZeneca plc, Merck KGaA, GlaxoSmithKline plc, Innovent Biologics, Inc., BeiGene, Ltd., Regeneron Pharmaceuticals, Inc., F. Hoffmann-La Roche Ltd., Shanghai Junshi Biosciences Co., Ltd., Bristol-Myers Squibb Company, Merck & Co., Inc. |
Analyst Review
The increase in demand for novel drugs and rise in investments for development of therapeutic agents for cancer globally are expected to offer profitable opportunities for the expansion of the market. In addition, favorable government initiatives and higher spending for cancer have piqued the interest of several companies to develop therapeutics for cancer treatment. Furthermore, increase in adoption of targeted drug therapy for cancer owing to enhanced efficacy, improved survival rates, and reduced toxicity as compared to conventional chemotherapy is expected to boost the growth of the market. In addition, personalized medicine approaches, on the basis of individual patient characteristics and tumor profiling have gained prominence in cancer treatment.Furthermore, North America accounted for the largest share in 2022 and is expected to remain dominant during the forecast period, owing to presence of large population suffering from various types of cancer, strong
presence of key players offering novel drugs, and favorable reimbursement policies in healthcare system. However, Asia-Pacific is anticipated to witness notable growth, owing to increase in investments for development of anti-cancer agents, surge in number of cancer cases, and rise in awareness related to available treatment options.
The total market value of immune checkpoint inhibitors market is $40,126.3 million in 2022.
The forecast period for immune checkpoint inhibitors market is 2023 to 2032
The market value of immune checkpoint inhibitors market in 2032 is $189,379.51 million.
The base year is 2022 in immune checkpoint inhibitors market.
Top companies such as Merck & Co., Inc., AstraZeneca plc, F. Hoffmann-La Roche Ltd., Merck KGaA and Regeneron Pharmaceuticals, Inc. held a high market position in 2022. These key players held a high market position owing to the strong geographical foothold in North America, Europe, Asia-Pacific, and LAMEA.
"The PD-1 inhibitor segment is the most influencing segment in immune checkpoint inhibitors market owing to rise in adoption of PD-1 inhibitors in various cancers, including melanoma, lung, and bladder cancers, owing to their superior efficacy and durable responses in treating cancers.
The major factor that fuels the growth of the immune checkpoint inhibitors market are increase in incidences of cancer across the globe, rise in geriatric population and supportive reimbursement policies for immune checkpoint inhibitors drive the growth of the global immune checkpoint inhibitors market .
Immune checkpoint inhibitors are a class of immunotherapy drugs that enhance the body's immune system to fight cancer. They work by blocking certain proteins, called checkpoints, that inhibit the immune response, allowing T cells to recognize and attack cancer cells more effectively.
Loading Table Of Content...
Loading Research Methodology...



