Inhaled Nitric Oxide Market Research, 2032
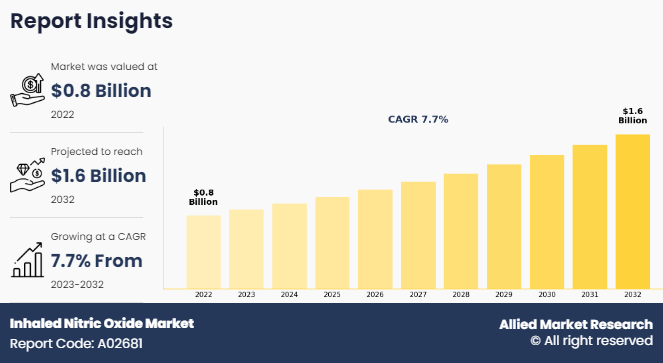
The global inhaled nitric oxide market was valued at $0.8 billion in 2022, and is projected to reach $1.6 billion by 2032, growing at a CAGR of 7.7% from 2023 to 2032. Rise in incidence of the preterm birth and high adoption of inhaled nitric oxide for treatment of respiratory complications in neonatal. According to a 2022 article by the National Library of Medicine, it was estimated that the rate of preterm births worldwide is 5–18% with the developing countries accounting for the maximum deaths. Furthermore, according to a 2022 article by the National Heart, Lung and Blood Institute, it was reported that premature birth, especially before 32 weeks of pregnancy, commonly leads to respiratory distress syndrome in newborns. In addition, the rise in the research and development in evaluation of the therapeutic effects of inhaled nitric oxide.
Key Takeaways
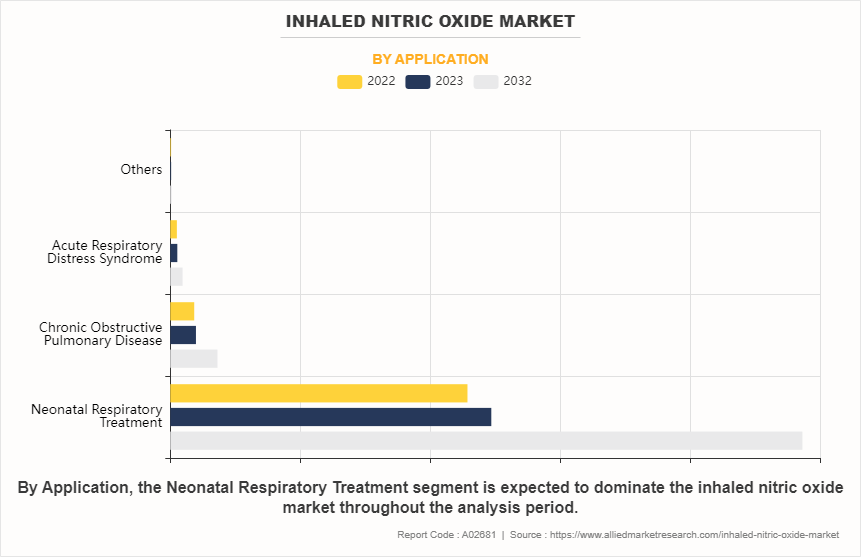
- By application, the neonatal respiratory treatment segment was the highest contributor to the inhaled nitric oxide market size in 2022.
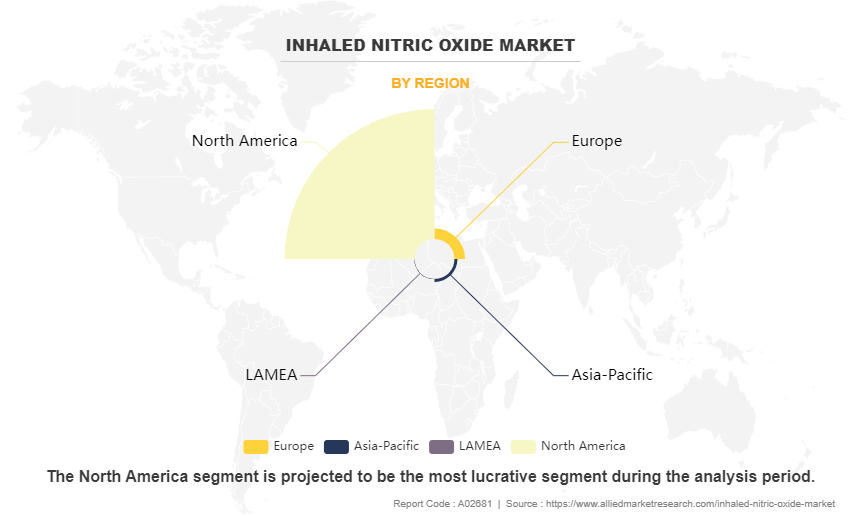
- By region, North America garnered the largest inhaled nitric oxide market share in 2022. However, Asia-Pacific is expected to grow at the fastest rate during the forecast period.
Inhaled nitric oxide is a therapeutic gas used in medicine primarily for its vasodilatory properties. It is a selective pulmonary vasodilator, meaning it specifically relaxes the blood vessels in the lungs without significantly affecting the systemic circulation. This selective action makes it a valuable tool in managing conditions such as persistent pulmonary hypertension in newborns (PPHN), acute respiratory distress syndrome (ARDS), and other forms of pulmonary hypertension.
Market Dynamics
The inhaled nitric oxide market growth is expected to rise significantly owing to rise in adoption of the inhaled nitric oxide for management of pulmonary disorders in the infants and newborns, surge in prevalence of respiratory disorders and development in healthcare infrastructure. In recent years, there has been a growing recognition of the efficacy of inhaled nitric oxide in treating various pulmonary conditions, particularly in the neonatal population. For instance, according to a 2022 article by the National Heart, Lung and Blood Institute, it was reported that, there are many types of breathing problems that affect newborns, such as transient tachypnea of the newborn, neonatal respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), meconium aspiration syndrome external link, persistent pulmonary hypertension of the newborn.
One of the primary indications for inhaled nitric oxide therapy is persistent pulmonary hypertension of the newborn (PPHN), a life-threatening condition characterized by elevated pulmonary vascular resistance. Inhaled nitric oxide works by selectively dilating the pulmonary vasculature, improving oxygenation, and reducing the need for invasive ventilation methods. Additionally, inhaled nitric oxide is approved by U.S. Food and Drug Administration for treatment of hypoxic respiratory failure. Thus, the high adoption of inhaled nitric oxide to treat respiratory complications in newborns is expected to drive inhaled nitric oxide market forecast.
Furthermore, the surge in incidence of respiratory diseases and air pollution has emerged as a significant inhaled nitric oxide market trends. In recent years, respiratory ailments such as chronic obstructive pulmonary disease (COPD), asthma, and acute respiratory distress syndrome (ARDS) have seen a notable rise in prevalence globally. Factors contributing to this surge include environmental pollution, lifestyle changes, and an aging population susceptible to respiratory conditions. In response, healthcare providers are increasingly turning to inhaled nitric oxide as a therapeutic intervention.
For instance, according to Australian Bureau of Statistics, it was estimated that in 2022, 2.5% (638,100) of people had COPD in Australia. Nitric oxide is known for its vasodilatory and anti-inflammatory properties, making it a promising treatment for various respiratory disorders. It helps improve oxygenation, reduce pulmonary hypertension, and alleviate symptoms associated with respiratory distress. According to an article by the National Library of Medicine, it was reported that inhaled nitric oxide can be clinically used for treatment of neonatal respiratory distress syndrome, pulmonary hypertension, and acute respiratory distress syndrome. Thus, the rise in prevalence of respiratory disorders is expected to drive the inhaled nitric oxide market forecast.
Moreover, the expansion and enhancement of healthcare infrastructure stand as pivotal drivers propelling the the inhaled nitric oxide market opportunity. Inhaled nitric oxide therapy has garnered significant attention for its therapeutic benefits in respiratory and cardiovascular disorders, particularly in conditions such as neonatal hypoxic respiratory failure and pulmonary hypertension. The expansion and modernization of healthcare infrastructure have facilitated greater accessibility to advanced medical technologies and treatments, including inhaled nitric oxide delivery systems. As the hospitals and healthcare facilities are now equipped with state-of-the-art infrastructure, they are better positioned to administer inhaled nitric oxide therapy promptly and efficiently, thereby improving patient outcomes.
The high cost of inhaled nitric oxide stands as a significant barrier within the inhaled nitric oxide market, impeding its widespread adoption and accessibility. Nitric oxide therapy is employed primarily in the treatment of conditions such as pulmonary hypertension and respiratory distress syndrome, where its vasodilatory properties offer therapeutic benefits. However, despite its efficacy, the substantial expense associated with producing, distributing, and administering inhaled nitric oxide remains a formidable obstacle. The complex manufacturing process, stringent quality control measures, and specialized delivery systems contribute to its elevated price point. This financial burden restricts access to nitric oxide therapy, particularly in regions with limited healthcare resources or constrained budgets.
Moreover, high research and development activities for inhaled nitric oxide is expected to provide significant growth inhaled nitric oxide market opportunity in the inhaled nitric oxide market. Inhaled nitric oxide has garnered attention for its potential therapeutic applications in various pulmonary and cardiovascular conditions, including respiratory distress syndrome in newborns, pulmonary hypertension, and chronic obstructive pulmonary disease (COPD). The continuous advancement in medical science and technology has propelled the exploration of novel uses and formulations of inhaled nitric oxide, leading to increased research and development investments by pharmaceutical companies and research institutions. These efforts aim to optimize delivery methods, dosage regimens, and expand the range of indications for inhaled nitric oxide therapy.
Segments Overview
The inhaled nitric oxide market analysis is segmented on the basis of application, and region. By application, the market is classified into neonatal respiratory treatment, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS), and others. Region-wise, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, and rest of Europe), Asia-Pacific (Japan, Australia, and rest of Asia-Pacific), and LAMEA (Latin America and Middle East and Africa).
By Application
The neonatal respiratory treatment segment dominated the global inhaled nitric oxide market share in 2022, owing to high adoption of the inhaled nitric oxide for treatment of respiratory disorders in newborns. Furthermore, the majority of the inhaled nitric oxide products are approved for treatment of respiratory disorders in neonatal. Neonatal respiratory conditions, such as persistent pulmonary hypertension of the newborn (PPHN) and respiratory distress syndrome (RDS), are critical medical issues that require immediate and specialized intervention. Inhaled nitric oxide has proven to be an effective therapeutic option for these conditions due to its ability to selectively dilate pulmonary vasculature, improving oxygenation and reducing pulmonary hypertension in newborns. As a result, there has been a growing demand for inhaled nitric oxide specifically for neonatal respiratory treatment.
By Region
The inhaled nitric oxide market is analyzed across North America, Europe, Asia-Pacific, LAMEA. North America accounted for a major inhaled nitric oxide market size in 2022 and is expected to maintain its dominance during the forecast period. This is attributed to various key factors such as including advanced medical facilities and a high level of healthcare spending, which facilitates the adoption of novel treatments like inhaled nitric oxide. In addition, extensive research and development activities, along with a supportive regulatory environment, have propelled the development and commercialization of inhaled nitric oxide products in North America. However, the Asia-Pacific region is expected to register the highest CAGR during the forecast period owing to high prevalence of respiratory conditions such as chronic obstructive pulmonary disease (COPD), pulmonary hypertension, and neonatal hypoxic respiratory failure in many parts of Asia-Pacific and increase in disposable income in the region.
Competitive Analysis
Competitive analysis and profiles of the major players in inhaled nitric oxide industry, such as Air Liquide S.A, Linde Plc, Mallinckrodt Plc, Vero Biotech LLC, Chemix Specialty Gases and Equipment, Air Water Inc, Sichuan Salman Chemical Products Co., Ltd., Linde PLC, SOL Group, Nippon Sanso Holdings Corporation and Chengdu Taiyu Industrial Gases Co., Ltd., are provided in this report. Major players have adopted patent and joint venture as key developmental strategies to improve the product portfolio of the inhaled nitric oxide market.
Recent Developments in Inhaled Nitric Oxide Industry
In April 2020, Linde PLC announced that the U.S. Supreme Court upheld the nitric oxide patent win for Linde. However, Linde supported the inhaled nitric oxide clinical studies relating to COVID-19. Further, Linde continues to supply inhaled nitric oxide. The product is approved and widely used in the U.S. and other countries to improve the oxygenation of certain groups of patients.
In January 2021, SOL strengthened its presence in the promising Indian technical and medical gases market by increasing its 85% stake in the Indian Joint Venture “SICGILSOL”. SICGILSOL was the first operator in India to make inhaled nitric oxide therapy available and the first to build a medical gas distribution system in accordance with ISO standards and those in practice in India.
Key Benefits For Stakeholders
This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the inhaled nitric oxide market analysis from 2022 to 2032 to identify the prevailing inhaled nitric oxide market opportunities.
The market research is offered along with information related to key drivers, restraints, and opportunities.
Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
In-depth analysis of the inhaled nitric oxide market segmentation assists to determine the prevailing market opportunities.
Major countries in each region are mapped according to their revenue contribution to the global market.
Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
The report includes the analysis of the regional as well as global inhaled nitric oxide market trends, key players, market segments, application areas, and market growth strategies.
Inhaled Nitric Oxide Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 1.6 billion |
| Growth Rate | CAGR of 7.7% |
| Forecast period | 2022 - 2032 |
| Report Pages | 179 |
| By Application |
|
| By Region |
|
| Key Market Players | Vero Biotech LLC, Mallinckrodt plc, Linde PLC, Chemix Specialty Gases and Equipment, Air Water Inc., Nippon Sanso Holdings Corporation, SOL Group, Sichuan Salman Chemical Products Co., Ltd, Chengdu Taiyu Industrial Gases Co., Ltd., Air Liquide S.A. |
Analyst Review
In recent years, the demand for inhaled nitric oxide has surged significantly, driven by its crucial role in the treatment of various respiratory conditions, particularly in neonates with persistent pulmonary hypertension. The inhaled nitric oxide market has witnessed a notable increase in adoption, as healthcare providers recognize the efficacy of inhaled nitric oxide therapy in improving oxygenation and reducing the need for more invasive interventions. Key factors contributing to the market's expansion include the rise in prevalence of respiratory diseases, advancements in medical technology, and increase in awareness among healthcare professionals regarding the benefits of inhaled nitric oxide.
The ongoing research and development activities in the pharmaceutical sector have also played a pivotal role in introducing innovative formulations and delivery systems, further enhancing the accessibility and efficacy of inhaled nitric oxide therapy. However, despite its promising prospects, the inhaled nitric oxide market faces challenges such as high treatment costs, regulatory hurdles, and limited accessibility in certain regions.
Inhaled nitric oxide is a therapeutic gas used in medicine primarily for its vasodilatory properties
The Inhaled Nitric Oxide Market growth is driven by rise in adoption of the inhaled nitric oxide for management of pulmonary disorders in the infants and newborns, surge in prevalence of respiratory disorders and development in healthcare infrastructure.
The neonatal respiratory treatment segment is the most influencing segment in the Inhaled Nitric Oxide Market.
Major key players that operate in the Inhaled Nitric Oxide Market are Mallindkroft Plc, Air Liquide, and Linde plc
The base year is 2022 in Inhaled Nitric Oxide Market
The market value of Inhaled Nitric Oxide Market in 2032 is expected to be $1.6 billion.
The forecast period for Inhaled Nitric Oxide Market is 2023-2032.
The total market value of Inhaled Nitric Oxide Market is $0.8 billion in 2022
Loading Table Of Content...
Loading Research Methodology...



