Intracranial Pressure Monitoring Market Research, 2033
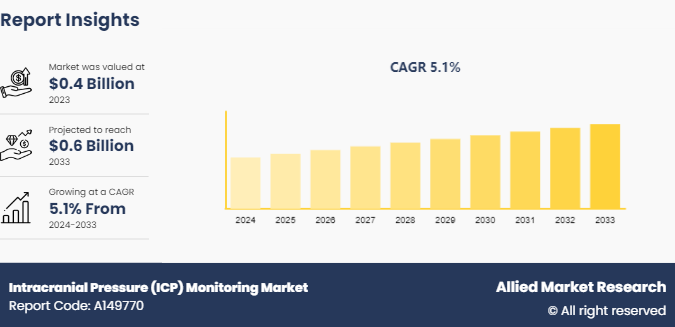
The global ICP monitoring market size was valued at $0.4 billion in 2023, and is projected to reach $0.6 billion by 2033, growing at a CAGR of 5.1% from 2024 to 2033.Increase in incidence of neurological disorders is driving the intracranial pressure monitoring market growth.
Market Introduction and Definition
Intracranial pressure (ICP) monitoring is a medical procedure used to measure the pressure inside the skull. This pressure is exerted by the brain, cerebrospinal fluid, and blood within the cranial cavity. Intracranial pressure monitoring involves the insertion of a device, such as a catheter or sensor, into the brain or cerebrospinal fluid space to directly measure the pressure.
ICP monitoring is crucial in various medical conditions, especially those involving traumatic brain injury, stroke, brain tumors, and other neurological disorders. Monitoring ICP helps healthcare providers assess the severity of brain injury, detect changes in intracranial dynamics, and guide treatment decisions. Elevated ICP can lead to serious complications such as brain herniation, decreased cerebral perfusion, and neurological deterioration. Timely detection and management of elevated ICP are essential to prevent secondary brain damage and improve patient outcomes. Therefore, intracranial pressure monitoring is important in optimizing patient care and minimizing the risk of long-term neurological impairment or mortality associated with intracranial hypertension.
Key Takeaways
- The intracranial pressure monitoring market share study covers 20 countries. The research includes a segment analysis of each country in terms of value for the projected period.
- More than 1, 500 product literatures, industry releases, annual reports, and other such documents of major ICP monitoring market participants along with authentic industry journals, trade associations' releases, and government websites have been reviewed for generating high-value industry insights.
- The study integrated high-quality data, professional opinions and analysis, and critical independent perspectives. The research approach is intended to provide a balanced view of global markets and to assist stakeholders in making educated decisions in order to achieve their most ambitious growth objectives.
Key Market Dynamics
The technological innovations in ICP monitoring devices have significantly improved accuracy, reliability, and patient comfort. Miniaturization of sensors, wireless connectivity, and integration with digital health platforms have enhanced the efficiency and effectiveness of ICP monitoring. These advancements drive market growth by enabling healthcare providers to obtain real-time data, make informed decisions, and optimize patient care in neurocritical settings.
However, despite technological advancements, the high cost associated with intracranial pressure monitoring devices poses a significant restraint to ICP monitoring market growth. The initial investment in acquiring monitoring systems, along with recurring costs for maintenance, calibration, and consumables, can be prohibitive for healthcare facilities, especially in resource-constrained settings. Cost constraints limit the adoption of ICP monitoring devices, particularly in low- and middle-income countries, thus hindering market expansion.
Moreover, increase in incidence of neurological disorders, such as traumatic brain injury (TBI) , stroke, intracranial hemorrhage, and brain tumors, serves as a significant opportunity for the intracranial pressure (ICP) monitoring market growth. As these conditions often result in elevated ICP, continuous monitoring is essential for timely detection and intervention to prevent secondary brain injury and optimize patient outcomes. With a growing population affected by neurological disorders globally, there is a heightened demand for advanced monitoring technologies such as ICP monitoring devices. This trend highlights the critical role of ICP monitoring in neurocritical care, thus driving market growth to meet the escalating healthcare needs.
Non-Invasive Methodologies Statistics of Global Intracranial Pressure Monitoring Market
Non-invasive intracranial pressure monitoring market trends methods offer alternatives to invasive procedures, providing valuable insights into ICP dynamics without the need for surgical intervention. These methods utilize various techniques, including neuroimaging, audiologic assessments, cerebral fluid dynamics analysis, electrophysiological measurements, ophthalmic evaluations, and other miscellaneous approaches. Among these, ophthalmic methods, which assess optic nerve sheath diameter and intraocular pressure, constitute the majority at 37.9%, reflecting their prominence in non-invasive ICP monitoring. Cerebral fluid dynamics analysis follows closely at 21.5%, indicating the importance of assessing cerebrospinal fluid dynamics in ICP management. Together, these non-invasive methodologies contribute to a comprehensive approach to ICP monitoring, facilitating timely interventions and optimizing patient care in neurocritical settings.
Non-Invasive Methodologies Statistics
Non-Invasive Methodologies | % (Percentage) |
Neuroimaging | 14.2% |
Audiological | 10.5% |
Cerebral Fluid Dynamics | 21.5% |
Ophthalmic | 37.9% |
Electrophysiological | 6.8% |
Miscellaneous | 9.1% |
Market Segmentation
The ICP monitoring market is segmented into technique, application, end user, and region. On the basis of technique, the market is bifurcated into invasive and non-invasive. On the basis of application, the market is divided into traumatic brain injury, intracerebral hemorrhage, meningitis and others. On the basis of end user, the market is divided into hospitals, clinics, private medical offices, trauma centers, and government and research organizations. By region, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Regional/Country Market Outlook
The regional and country market outlook for the intracranial pressure monitoring market share varies significantly based on factors such as healthcare infrastructure, prevalence of neurological disorders, regulatory landscape, and reimbursement policies. In developed regions such as North America and Europe, where there is a robust healthcare system and high prevalence of neurological conditions, the market for ICP monitoring devices is well-established.
Emerging economies in Asia-Pacific and Latin America are witnessing rapid growth in intracranial pressure monitoring market forecast, driven by increase in healthcare expenditure, improvement in healthcare infrastructure, and rise in awareness about neurocritical care. Countries such as China, India, and Brazil are expected to contribute significantly to market growth in these regions due to their large population bases and growth in burden of neurological disorders. However, challenges such as limited access to healthcare services and regulatory hurdles may hinder market growth in certain regions. Overall, the regional and country market outlook for the ICP monitoring market reflects a complex interplay of factors influencing demand and adoption of these critical medical devices.
- In February 2023, NovaSignal Corporation announced that it has donated the NovaGuide 2 Intelligent Ultrasound to the Jacobs Institute. The Jacobs Institute continues research on the use of transcranial Doppler (TCD) in continuous neuromonitoring to improve neurovascular procedures and minimize the risk of stroke.
- In 2024, Natus Medical has developed the new generation of the Camino Intracranial Pressure and Temperature Monitor, offering rapid ICP assessment while easing stress for clinicians during critical moments.
Industry Trends
- In April 2024, The Brain Trauma Foundation (BTF) and the Military Traumatic Brain Injury Initiative (MTBI2) announced their collaboration for multicenter randomized, phase one, clinical trial and the development of three new clinical TBI practice guidelines. A successful, previous collaboration between the MTBI2 and the BTF to produce new penetrating brain injury guidelines fostered this recent collaboration.
- The American Society of Craniofacial Surgery (ASCFS) primarily focuses on craniofacial surgical interventions for congenital and acquired craniofacial anomalies. ASCFS surgeons may collaborate with neurosurgeons to assess and manage elevated ICP, aiming to optimize patient outcomes. While ASCFS guidelines primarily address craniofacial surgical techniques and principles, understanding the implications of elevated ICP and its management is essential for comprehensive craniofacial care.
- In April 2023, the third edition of the Brain Trauma Foundation’s evidence-based guidelines for the prehospital management of traumatic brain injury (TBI) was published in Prehospital Emergency Care.
Competitive Landscape
The major players operating in the ICP monitoring market size include AMETEK, Elekta AB, Koninklijke Philips N.V., Smiths Medical, Sick AG, Teleflex Incorporated, GE Healthcare, Integra LifeSciences, Natus Medical Incorporated, BrainLAB. Other players in intracranial pressure monitoring market include Medtronic PLC, Codman, and Shurtleff inc., Sophysa Ltd., Orsan Medical Technologies, Raumedic AG and so on.
Recent Key Strategies and Developments
- In January 2023, Natus Medical Incorporated, a leading provider of medical device solutions focused on the screening, diagnosis, and treatment of central nervous and sensory system disorders, announced the closing of its acquisition of Micromed Holding SAS ("Micromed") , a global provider of neurophysiology solutions. This newly merged organization brings together innovative neurodiagnostic and neuromonitoring solutions and experienced teams to offer a broader portfolio of products, services and support to customers worldwide.
- In August 2020, IRRAS AB, with a broad portfolio of innovative products for neurocritical care, announced the first patient treatment with Hummingbird Solo, a new line extension to its innovative Hummingbird ICP Monitoring product family.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the intracranial pressure monitoring market analysis from 2024 to 2033 to identify the prevailing intracranial pressure monitoring market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the intracranial pressure monitoring market segmentation assists to determine the prevailing ICP monitoring market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global ICP monitoring market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the ICP monitoring marketplayers.
The report includes the analysis of the regional as well as global intracranial pressure monitoring market trends, key players, market segments, application areas, and market growth strategies.
Key Sources Referred
- World Health Organization (WHO)
- Brain Injury Association of America (BIAA)
- Centers for Medicare & Medicaid Services (CMS)
- Brain Trauma Foundation (BTF)
- National Health Service (NHS)
- Australian Government Department of Health and Aged Care
- Government of Canada's Health and Wellness
- Ministry of Health and Family Welfare (MoHFW)
- National Health Mission (NHM)
- Ayushman Bharat - Health and Wellness Centers (AB-HWCs)
- Centers for Disease Control and Prevention (CDC)
- Food and Drug Administration (FDA)
- National Institutes of Health (NIH)
Intracranial Pressure (ICP) Monitoring Market Report Highlights
| Aspects | Details |
| Market Size By 2033 | USD 0.6 Billion |
| Growth Rate | CAGR of 5.1% |
| Forecast period | 2024 - 2033 |
| Report Pages | 216 |
| By Technique |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | Integra LifeSciences, Natus Medical Incorporated, AMETEK, Koninklijke Philips N.V, BrainLAB, Smiths Medical, Sick AG, Elekta AB, Teleflex Incorporated, GE Healthcare |
Loading Table Of Content...



