Medical Device Regulatory Affairs Market Research, 2031
The global medical device regulatory affairs market size was valued at $7.0 billion in 2021, and is projected to reach $12.2 billion by 2031, growing at a CAGR of 5.8% from 2022 to 2031. Medical device regulatory affair is a government affair, which is a specialty in regulated industries such as pharmaceuticals, medical devices, and agrochemicals. Within the healthcare industry, regulatory affairs have a very specific connotation.

The regulatory function in the healthcare industry is critical in ensuring the availability of safe and effective healthcare products around the world. Regulatory professionals include individuals who ensure regulatory compliance and prepare submissions, as well as those whose primary job function is clinical affairs or quality assurance. Medical device regulatory affairs experts serve as a link between the medical device industry and regulatory bodies around the world, including the United States Food and Drug Administration (USFDA), Medicines and Healthcare Products Regulatory Agency (MHRA) for UK, European Medicine Agency for the European Union, Central Drugs Standard Control Organization (CDSCO) for India, Pharmaceutical & Food Safety Bureau (PFSB) for Japan, and Therapeutic Good Administration (TGA) for Australia.
A novel medical device might cost millions to develop, and any error has a significant influence on the company's reputation. As medical devices play such an important role in people's lives, it will help in diagnosis, prevention and treatment of various diseases so it is important to check the quality of the product/medical devices and for this purpose the regulatory affairs expert is fully responsible for keeping products in conformity and keeping track of all paperwork. One of the most important responsibilities of the regulatory specialist is to guarantee that all information about device is accurately communicated to patients. Even a minor blunder in any regulatory activity can result in the product being recalled, as well as loss of millions of dollars.
The market is expected to witness a moderate growth during the forecast period, owing to rise in adoption and development of advanced medical devices for the treatment of various diseases such as cardiovascular, cancer, and other infectious diseases, along with various technological advancements and their increased applications in the healthcare sector. Furthermore, surge in geriatric population along with various technological advancements in the market to meet the unmet needs of patients provides significant opportunities for existing players and new entrants.
However, factors such as high cost of providing regulatory services and increase in number of cyberattacks for software based medical devices are expected to hamper growth of the market up to some extent during the forecast period.
The medical device regulatory affairs market size is segmented into Services, Service Provider, Types and Indication. On the basis of services, the market is divided into regulatory consulting /strategic services, regulatory writing & publishing, legal representation, product registration & clinical trials, and others. By service provider, it is classified into in-house and outsourcing. The type segment includes diagnostics and therapeutics. Depending on indication, the market is fragmented into infectious diseases, oncology & hematology, gynecology & obstetric, musculoskeletal disorder, respiratory, cardiovascular and others. Region wise, it is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
Services Segment review
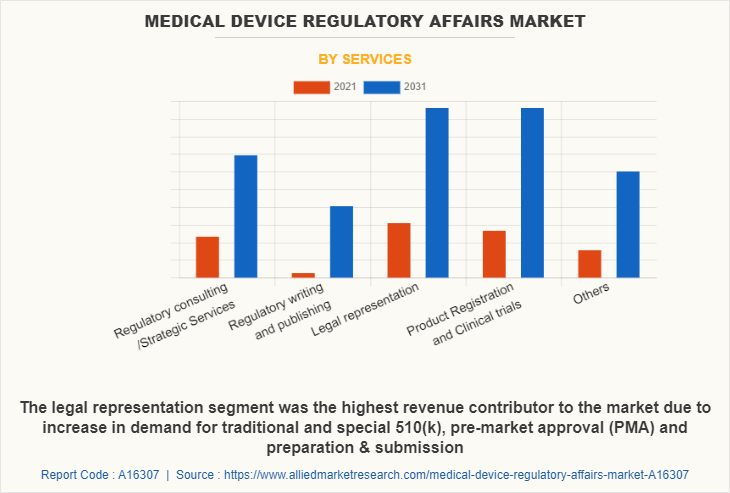
By services segment, the medical device regulatory affairs market is divided into regulatory consulting /strategic services, regulatory writing & publishing, legal representation, product registration & clinical trials, and others. The legal representation segment was the highest revenue contributor to the market, in 2021, and is estimated to register a CAGR of 6.1%. Owing to increase in demand for traditional and special 510(k), pre-market approval (PMA) preparation & submission, design dossier preparation & FDA notified body, and ISO 13485 registrar audit preparation & participation accessibility are expected to drive the market growth.
The product registration and clinical trials segment is estimated to reach at a significant CAGR of 6.8% during the medical device regulatory affairs market forecast period. Owing to rising demand for faster approval processes.
Service Provider Segment Review
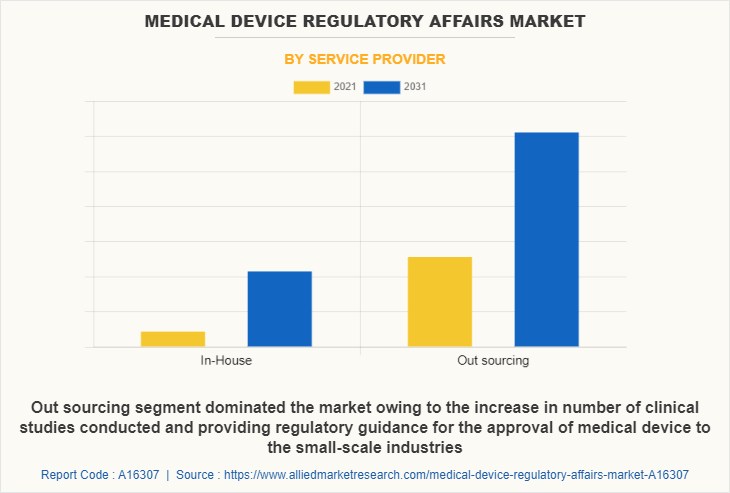
By service provider, the outsourcing segment was the highest revenue contributor to the market and is estimated to register a CAGR of 6.0%. The increase in number of clinical studies conducted and providing regulatory guidance for the approval of medical device to the small-scale industries act as a driver to boost the growth of the market.
The in-house segment is estimated to reach at a significant CAGR of 5.5% during the forecast period. Owing to the increasing complexity of medical devices and increase in demand for regulatory services required by small scale industries to get their product approval globally also because of the advantages such as cost and time saving.
Types Segment Review
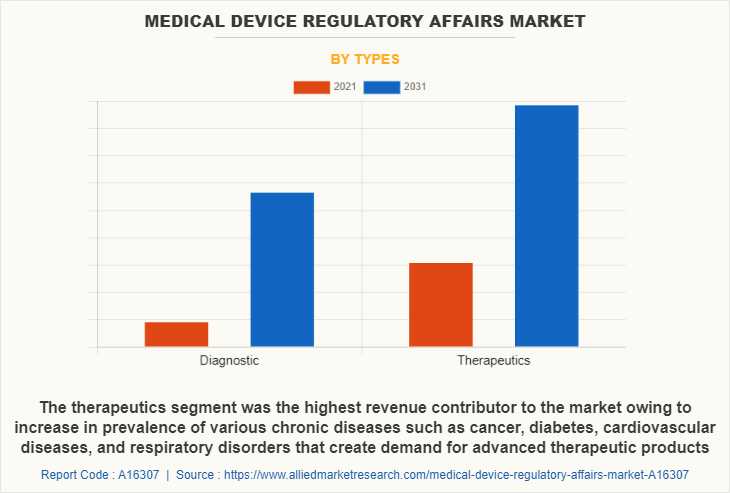
By type, the therapeutics segment was the highest revenue contributor to the medical device regulatory affairs industry with a CAGR of 5.6%. Owing to increase in prevalence of various chronic diseases such as cancer, diabetes, cardiovascular diseases, and respiratory disorders that create demand for advanced therapeutic products.
For instance, increasing demand for technologically advanced products such as auto-injectors or pen needles for effective and efficient delivery of insulin in diabetic patients is driving the segment.
The diagnostic segment is estimated to reach a significant CAGR of 6.2% during the forecast period. Due to a lack of supply, temporary approvals for expired therapeutic equipment such as respirators/ventilators and certification of similar equipment such as positive pressure breathing devices and anesthesia gas machines are permitted.
Indication Segment Review
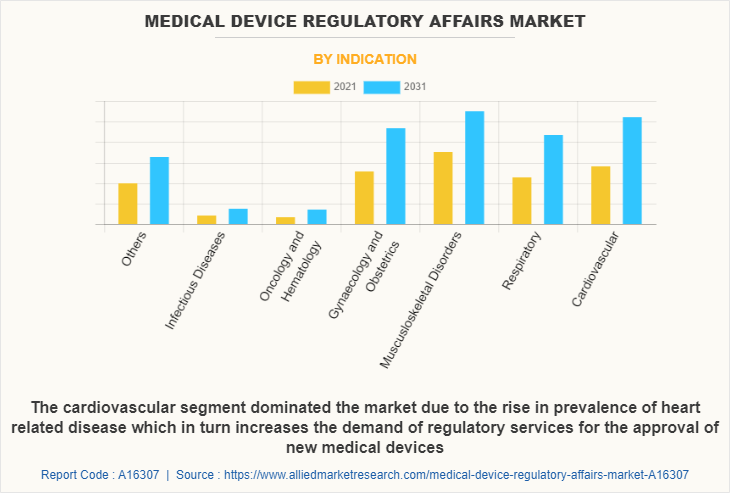
On the basis of indication, the musculoskeletal disorders segment was the highest revenue contributor to the market, with a CAGR of 4.6%. Due to increase in demand of advanced medical devices for oncology & hematology, the demand for the regulatory service required also increases which drives the growth of the market.
The cardiovascular segment is estimated to a significant CAGR of 6.5% during the forecast period. Owing to rise in prevalence of heart related disease which increase the demand of regulatory services for the approval of new medical devices.
Region Segment Review
Region wise, the medical device regulatory affairs market share is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America was the largest shareholder in the medical device regulatory affairs market in 2021. Increase in geriatric population, R&D investments, and supportive government initiatives directed toward use of advanced medical devices for chronic diseases are the major factors that drive growth of the medical device regulatory affairs market.
Asia-Pacific medical device regulatory affairs market analysis is estimated to grow during the forecast period. Rise in number of product approval, increase in number of clinical trials procedures, surge in demand for regulatory services emphasis of prominent players in enhancing their presence and high demand for advanced medical devices in the region.
Key players operating in the global medical device regulatory affairs industry include Amerisource Bergen, Charles River, Cliniexpert, Emergo, Icbio, Icon PLC, IQVIA, NKG, Parexel and Pepgra.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the medical device regulatory affairs market analysis from 2021 to 2031 to identify the prevailing medical device regulatory affairs market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the medical device regulatory affairs market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global medical device regulatory affairs market trends, key players, market segments, application areas, and medical device regulatory affairs market growth strategies.
Medical Device Regulatory Affairs Market Report Highlights
| Aspects | Details |
| By Services |
|
| By Service Provider |
|
| By Type |
|
| By Indication |
|
| By Region |
|
| Key Market Players | Pepgra, Emergo, IQVIA Holdings Inc., Amerisource Bergen, parexel, icbio cro, Charles river, NKG, Clini expert, icon plc |
Analyst Review
In accordance to several interviews conducted, the medical device regulatory affairs market is expected to witness a significant growth in the future, owing to benefits of regulatory services such as maintaining a smart quality system, including design controls to meet FDA, EU, and ISO 13485 requirements, among other qualifications and navigate the regulatory and development landscape while sticking to budgets and timelines.
According to perspectives of CXOs, the global medical device regulatory affairs market is expected to witness a steady growth in the future. The global medical device regulatory affairs market is progressing well as demand for faster approval processes grows. The need for the global medical device regulatory affairs market is being boosted by the rise of developing industries such as clinical research organization and pharmaceuticals.
However, factors such as several additional regulatory difficulties, including medical device and in vitro diagnostic device regulation, as well as regulatory cybersecurity monitoring are complicating, these are expected to hamper growth of the market during the forecast period. On the contrary, rise in technological advancements and increase in government initiatives for medical device regulations are expected to drive growth of the global medical device regulatory affairs market.
The global Medical Device Regulatory Affairs Market was valued at $6,969.7 million in 2021, and is projected to reach $12,247.7 million by 2031, registering a CAGR of 5.8% from 2022 to 2031.
North America was the largest shareholder in the medical device regulatory affairs market in 2021. Increase in geriatric population, R&D investments, and supportive government initiatives directed toward use of advanced medical devices for chronic diseases are the major factors that drive growth of the medical device regulatory affairs market.
Few trends such as the increase in R&D in developing medical devices especially diagnostic medical devices
Amerisource Bergen, Charles River, Icon Plc and Iqvia are few players to hold major share in the market
Loading Table Of Content...



