Methanol Catalyst Market Research, 2032
The global methanol catalyst market was valued at $6.0 billion in 2022, and is projected to reach $8.0 billion by 2032, growing at a CAGR of 3.2% from 2023 to 2032.
Report Key Highlighters:
- The report provides competitive dynamics by evaluating business segments, product portfolios, target market revenue, geographical presence and key strategic developments by prominent manufacturers.
- The methanol catalyst market is fragmented in nature among prominent companies such as Air Liquide Engineering & Construction, BASF SE, Casale SA, CLARIANT, Johnson Matthey, MITSUBISHI GAS CHEMICAL COMPANY, INC., Sinopec Catalyst CO., LTD., Smart Catalyst, Süd-Chemie India Pvt. Ltd. and Topsoe.
- The study contains qualitative information such as the market dynamics (drivers, restraints, challenges, and opportunities), key regulation analysis, pricing analysis, and Porter’s Five Force Analysis across North America, Europe, Asia-Pacific, LAMEA regions. Moreover, the report covers sub-segments that is studied across all the regions.
- Latest trends in global methanol catalyst market such as undergoing R&D activities, regulatory guidelines, and government initiatives are analyzed across 16 countries in 4 different regions.
A methanol catalyst is a substance that facilitates the chemical reaction that converts a mixture of carbon monoxide and hydrogen gas (known as syngas) into methanol. A catalyst is a material that increases the rate of a reaction without being consumed in the process. Methanol is a significant chemical used as a fuel, a solvent, and a feedstock for the synthesis of other chemicals. Methanol production in industry depends heavily on methanol catalysts. The most common types of methanol catalysts are copper-based catalysts, which typically contain copper, zinc oxide, and aluminum oxide. The Haldor Topsoe process, which is characterized by high pressure and low temperature, is frequently combined with these catalysts. Other types of methanol catalysts include iron-based catalysts and mixed metal oxide catalysts.
The wide usage of methanol in various end-use industries including automobile and pharmaceutical drives the growth of the methanol catalyst market. Methanol is used as an alternative fuel in the automotive sector, since it is less expensive and burns cleaner than conventional fossil fuels such as petrol and diesel. Methanol can be used in cars either as a pure fuel or in a fuel blend with other fuels including petrol.
Dimethyl ether (DME), a product of the conversion of methanol, is a diesel replacement. Lower greenhouse gas emissions are one of the key benefits of utilizing methanol as fuel. It burns cleaner than petrol and produces less harmful pollutants such as nitrogen oxides and carbon monoxide. Additionally, because methanol has a greater octane rating than petrol, it may be utilized in high-performance engines without endangering the engines or knocking them. It is a potentially sustainable and low-carbon fuel choice because it is produced from several renewable sources, including biomass, municipal trash, and carbon dioxide.
Moreover, it can be used in fuel cells to provide electricity for electric vehicles. In this application, methanol is transformed into hydrogen, which is then utilized to fuel the fuel cell and generate electricity to power the car. Although this technology is still in its initial stages, it has the potential to offer an improved and cleaner power source for electric vehicles.
Methanol is used in the pharmaceutical industry in numerous ways, including as a solvent and as a raw ingredient. Some APIs (active pharmaceutical ingredients), such as antibiotics and antifungal medicines, are made using methanol as a raw ingredient. These substances can be obtained from natural sources and extracted and purified using methanol, or they may be produced chemically. Some medicine formulations, such as those for injectable medications and oral suspensions, use methanol as a solvent.
The bioavailability of the active components in these formulations can be increased with methanol. In analytical chemistry, methanol is frequently employed as a solvent for the separation and examination of medicinal substances. Methanol is a polar solvent that can dissolve a variety of organic molecules, making it beneficial for analytical methods including spectrophotometry and chromatography.
Furthermore, methanol is employed as a cleaning and sanitizing agent. In pharmaceutical manufacturing facilities, equipment and surfaces can be cleaned with methanol, and it can also be used to sterilize medical equipment and instruments. Owing to the wide range of applications of methanol in the automotive and pharmaceutical sectors, the demand for methanol catalyst market will increase significantly during the forecast period.
The development of new catalysts for methanol generation is another factor that drives the growth of the market. The production of methanol can be achieved by using bimetallic catalysts, which combine the catalytic properties of two distinct metals. For instance, it has been demonstrated that platinum-rhenium catalysts are more active and selective than conventional copper-zinc oxide-aluminum oxide catalysts.
Rhenium has a special electronic structure and coordination environment, which has been discovered to improve the catalytic characteristics of platinum-based catalysts when rhenium is added to them. Rhenium may give electrons to the platinum atoms because it has a high electron density, which increases the catalyst's activity. Additionally, the addition of rhenium might help in lowering the production of undesirable byproducts such as methane and higher alcohols, which may lower the reaction's selectivity.
Zeolite-based catalysts are also used to produce methanol due to its special characteristics. Zeolites are porous substances with a distinct crystal structure and a large surface area that can function as catalysts. One of the advantages of using zeolite-based catalysts for methanol generation is that they can be tailored to provide specific properties, such as pore size and acidity, which can affect the catalytic activity and selectivity.
For instance, zeolites such as ZSM-5 and beta zeolites, which have various pore sizes and shapes, are employed to synthesize methanol. The effectiveness of the reaction may be impacted by the structure of the zeolite, which may affect how readily reactants and products diffuse inside the catalyst.
Furthermore, a novel kind of catalysis called plasma-assisted catalysis uses plasma to activate the catalyst and increase its activity. To produce methanol, the use of plasma-assisted copper-zinc oxide-aluminum oxide catalysts, which have showed encouraging results in terms of increasing process efficiency. Owing to these factors, the use of new catalysts for methanol generation boosted the growth of the market.
The high capital investment to produce syngas, which is a raw material for methanol synthesis restrains the markets’ growth. The generation of syngas, which is a mixture of hydrogen and carbon monoxide, often requires a substantial investment in capital equipment due to the complexity of the process and the requirement for high-temperature and high-pressure conditions. Specialized reactors with elevated temperature and pressure capabilities are necessary for the gasification of feedstocks such as coal, natural gas, or biomass. High-quality materials might be needed for these reactors to sustain the challenging operating circumstances, which might make them expensive to design, build, and operate.
Additionally, impurities in the syngas that result from gasification, such as sulfur compounds, particle matter, and tar, have the potential to harm downstream machinery and decrease process efficiency. Gas cleaning and conditioning systems are therefore necessary to get rid of these impurities and get the syngas ready for further processing. It can be expensive to build, run, and maintain these systems. Also, the low concentration of hydrogen in the combination makes it difficult to separate and purify the hydrogen from the syngas. The complexity of the separation and purification systems and the need for specialized materials, like membranes or adsorbents, might raise the capital cost. Owing to these factors, the growth of methanol catalyst market is hindered.
Methanol is typically produced via the reaction of hydrogen (H2) and carbon dioxide (CO2) in the presence of a catalyst. However, since CO2 is a major factor in climate change, the manufacture of methanol from CO2 can also contribute to greenhouse gas emissions. There are several methods that can be used to mitigate CO2 emissions in methanol synthesis which is an excellent opportunity for the methanol catalyst market.
Carbon capture and storage (CCS) approach involves capturing CO2 from the methanol synthesis process and storing it in geological formations like deep salt aquifers or depleted oil and gas reservoirs. CCS can drastically reduce the quantity of CO2 released into the atmosphere and lessen the negative effects that methanol production has on the environment.
Moreover, CO2 emissions from industrial activities, such as electricity generation or cement manufacturing, can be captured and used as a feedstock for the synthesis of methanol. This strategy can support the circular economy and lessen the quantity of CO2 that is released into the atmosphere. Many key players adopted various key strategies to decrease CO2 emissions.
For instance, in October 2021, Johnson Matthey (JM) and Carbon Recycling International (CRI) collaborated to supply JM’s KATALCO methanol catalysts in CRI’s Emissions-To-Liquids (ETL) designed CO2 to methanol plants. This collaboration will help meet the urgent need to remove carbon emissions in hard-to-decarbonize sectors of the economy. Owing to these factors, the mitigation of CO2 is a lucrative opportunity for the methanol catalyst market.
The methanol catalyst market is divided on the basis of type, application and region. On the basis of type, the market is classified into copper-based catalysts, zinc-based catalysts and others. Depending on the application, the market is segregated into industrial field, automobile field and others. Region wise, the market is analyzed across North America, Europe, Asia-Pacific and LAMEA.
The key players operating in the global methanol catalyst market are Air Liquide Engineering & Construction, BASF SE, Casale SA, CLARIANT, Johnson Matthey, MITSUBISHI GAS CHEMICAL COMPANY, INC., Sinopec Catalyst CO., LTD., Smart Catalyst, Süd-Chemie India Pvt. Ltd. and Topsoe. These players have adopted various key strategies including agreement, collaboration, expansion, and partnership to increase their market shares.
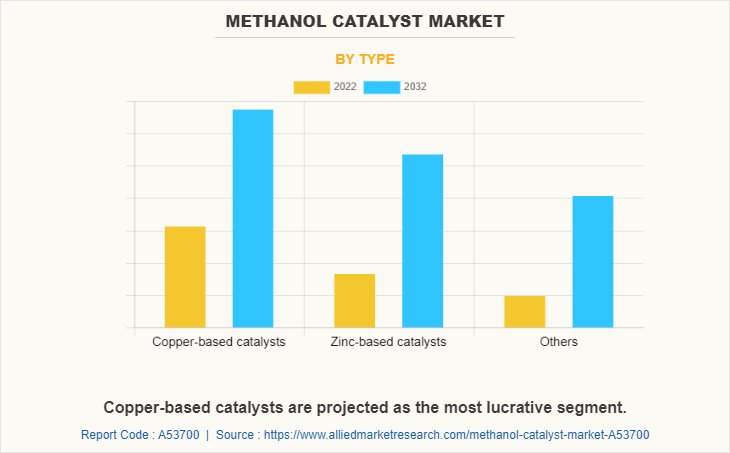
The copper-based catalyst segment is the leading by type, due to its wide application in various end-use industries including automobile, chemical, polymer industry, and others. Copper-based catalysts are used extensively due to their excellent properties such as cost effectiveness, versatility, high catalytic activity and their ability to maintain their catalytic activity over a prolonged period.
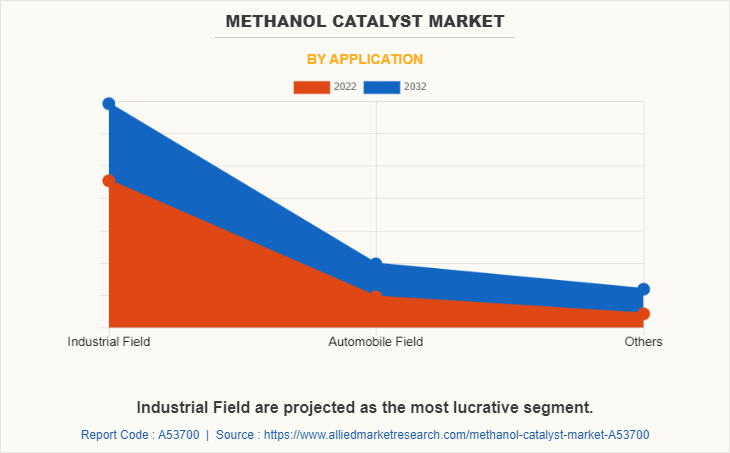
The industrial field segment dominated the global market, because of the growing demand for methanol catalyst in different sectors including petrochemicals, refining, automobile, and energy. Methanol is considered as a clean fuel as it has less environmental impact and decreases CO2 emissions.
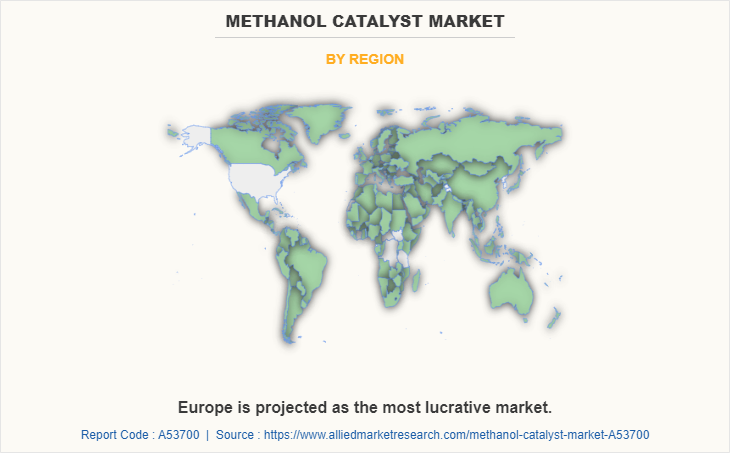
Region wise, Europe dominated the market as the region has significantly seen a rise in various foreign investments in the automobile, construction, and energy sectors which boosted the demand for methanol catalyst. Also, the surge in research and development activities expanded the methanol catalyst market in this region.
Key Developmental Strategies Undertaken by Key Players
In October 2021, Johnson Matthey (JM) and Carbon Recycling International (CRI) collaborated to supply JM’s KATALCO methanol catalysts in CRI’s Emissions-To-Liquids (ETL) designed CO2 to methanol plants. This collaboration will help meet the urgent need to remove carbon emissions in hard-to-decarbonize sectors of the economy.
In June 2021, Air Liquide Engineering & Construction continued its partnership with Samsung Engineering to build a methanol production plant for Sarawak Petchem, a state-owned oil and gas firm established and owned by the State Government of Sarawak, Malaysia. The methanol plant will produce 5,000 tons of methanol per day based on Air Liquide Engineering & Construction proprietary process technology, Lurgi MegaMethanol, which converts natural gas into methanol. This partnership will stimulate the demand for methanol catalyst market.
In March 2021, Johnson Matthey (JM) announced an agreement to supply innovative technologies and equipment to the world’s first methanol plant to harness energy from the wind, the Haru Oni project in Patagonia, Chile. The Haru Oni project, which is being developed by Siemens Energy in collaboration with Johnson Matthey and several other major companies, including Porsche, will be the first integrated and commercially successful large-scale facility in the world to manufacture climate neutral e-methanol and e-gasoline. This agreement will increase the demand for the methanol catalyst market.
In May 2020, Topsoe and Air Products signed a global alliance agreement for collaboration on large-scale ammonia, methanol, and/or dimethyl ether projects around the globe. In accordance with the alliance agreement, Air Products will be able to utilize Topsoe's technology license(s) and will be given access to specific engineering design, equipment, high-performance catalysts, and technical services for ammonia, methanol, and/or dimethyl ether plants that it will construct, own, and operate. Through the agreement, Topsoe's technology can be included into other Air Products technologies, including those for the gasification of diverse feedstocks and the production of synthesis gas. This agreement will boost the demand for the methanol catalyst market.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the methanol catalyst market analysis from 2022 to 2032 to identify the prevailing methanol catalyst market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the methanol catalyst market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global methanol catalyst market trends, key players, market segments, application areas, and market growth strategies.
Methanol Catalyst Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 8 billion |
| Growth Rate | CAGR of 3.2% |
| Forecast period | 2022 - 2032 |
| Report Pages | 320 |
| By Type |
|
| By Application |
|
| By Region |
|
| Key Market Players | CLARIANT, BASF SE, MITSUBISHI GAS CHEMICAL COMPANY, INC., Topsoe, Casale SA, Süd-Chemie India Pvt. Ltd., Sinopec Catalyst CO., LTD., Air Liquide Engineering & Construction, Smart Catalyst, Johnson Matthey |
Analyst Review
According to the opinions of various CXOs of leading companies. Methanol catalyst is used to facilitate the conversion of hydrogen and carbon monoxide into methanol, often by a procedure known as the methanol synthesis reaction. The growth of methanol catalyst is significantly attributed to the increased usage of methanol in various end-use industries including healthcare, electronics, automobile, and polymer industries, and developments in the new catalyst used for methanol generation. However, high capital cost to produce syngas, which is a raw material for the generation of methanol is expected to pose a threat to the methanol catalyst market. Furthermore, the fluctuations in the prices of methanol hamper the growth of this market. The mitigation of CO2 emissions has led to excellent growth opportunities for many manufacturers across the globe.
Currently, copper-based catalysts are the most used catalyst; however, zinc-based catalysts are expected to grow in terms of revenue. Similarly, the Asia-Pacific region is expected to grow at a significant rate during the forecast period.
The industry is characterized by a high number of new market entrants that seek to tap lucrative opportunities in the global market while existing players enter strategic collaborations to increase capacities & expand their reach into emerging markets. The agreement, collaboration, and expansion activities in the industry have increased significantly over the past decade. Companies constantly seek to establish long-term contract agreements with trusted partners for sustainable business operations globally.
Catalyst manufacturers are continually investing in research and development to enhance catalyst performance. This includes developing catalyst formulations with improved selectivity, higher activity, and longer catalyst lifetimes. Catalyst technology advancements, such as the development of bimetallic or multifunctional catalysts, are being explored to optimize methanol production processes.
Industrial Field application is the potential customers of Methanol Catalyst Market industry.
Asia-Pacific region will provide more business opportunities for Methanol Catalyst market in coming years.
The market players are adopting various growth strategies and also investing in R&D extensively to develop technically advanced unique products which are expected to drive the market size.
BASF SE, Casale SA, CLARIANT, Johnson Matthey, MITSUBISHI GAS CHEMICAL COMPANY, INC., Sinopec Catalyst CO., LTD., Smart Catalyst, Süd-Chemie India Pvt. Ltd. and Topsoe are the top players in Methanol Catalyst market.
Loading Table Of Content...
Loading Research Methodology...



