Multiplexed Diagnostics Market Research, 2032
The global multiplexed diagnostics market size was valued at $10.7 billion in 2022, and is projected to reach $41.5 billion by 2032, growing at a CAGR of 14.5% from 2023 to 2032. Multiplexing is the process of simultaneously detecting or identifying multiple biomarkers in a single diagnostic test, which can be valuable for several different types of diseases. In addition, the ability to simultaneously detect and identify the most frequent causes of infectious diseases directly from clinical specimens is useful for patient care, hospital infection control practices, and epidemiologic studies. A variety of commercially available nucleic acid amplification platforms can target multiple microorganisms in a single test reaction. This approach is being increasingly applied for diagnosing various infectious diseases.

Market Dynamics
The increase in the prevalence of chronic disease such as infectious disease, cardiac disorders, respiratory and allergic diseases, and various others increases the demand for the precise diagnosis for the analysis of multiple biomarkers or analytes in a single assay, reducing the time and resources required compared to performing individual tests are the factors that propel the growth of the multiplexed diagnostics market size. In addition, surge in demand for personalized medicine due to change in shift from the conventional medical treatment to personalized, or precision, medicine, and rise in technological advancements for the development of innovative technologies such as microarrays, bead-based assays, and next-generation sequencing has expanded the possibilities for multiplexed diagnostics are the major factors that propel the growth of the market.
Moreover, the increase in the number of key market players that provide multiplexed diagnostics tools and kits and the strategies they adopt to improve the product portfolio of the multiplexed diagnostics are the factors that propel the growth of the market. For instance, in January 2023 Agilent Technologies, Inc. announced a partnership with Akoya Biosciences, Inc. to develop multiplex-immunohistochemistry diagnostic solutions for tissue analysis and to commercialize workflow solutions for multiplex assays in the clinical research market. Integrating Dako Omnis (auto staining instrument) of Agilent and PhenoImager HT (imaging platform) of Akoya for multiplex chromogenic immunohistochemistry (mIHC) and immunofluorescent (mIF) assays is expected to create a singular end-to-end commercial workflow, including reagents, staining, imaging, and analysis.
Furthermore, the role of multiplexed diagnostics in drug development and clinical trials by providing insights into biomarker profiles, patient stratification, and treatment response, can accelerate the development of new therapies. Thus, increase in the number of R&D activities globally and increase in collaborations between diagnostic companies, pharmaceutical companies, and research institutions have led to the development of multiplexed assays with both diagnostic and therapeutic applications, are the factors that provide lucrative opportunities for the growth of the market.
Segmental Overview
The multiplexed diagnostics market share is segmented into product type, application, end user, and region. On the basis of product type, the multiplexed diagnostics market share is bifurcated into instruments & accessories and kits & reagents. Based on application, it is segregated into infectious disease, oncology, autoimmune disease, cardiac disease, allergies, and others. Based on end user, it is segmented into hospitals, diagnostic centers, and others. On the basis of region, it is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and rest of Europe), Asia-Pacific (China, Japan, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
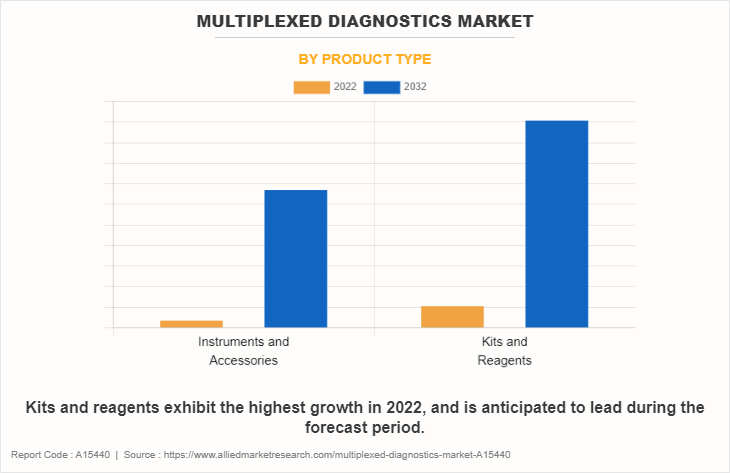
By Product Type
The multiplexed diagnostics market is segmented into instruments & accessories and kits & reagents. The kits & reagents segment generated maximum revenue in 2022, owing to the increased awareness & acceptance of multiplexed diagnostics kits among the researchers and cost savings by opting for multiplexed diagnostics. However, the instruments & accessories segment is expected to witness a significant CAGR during the forecast period.
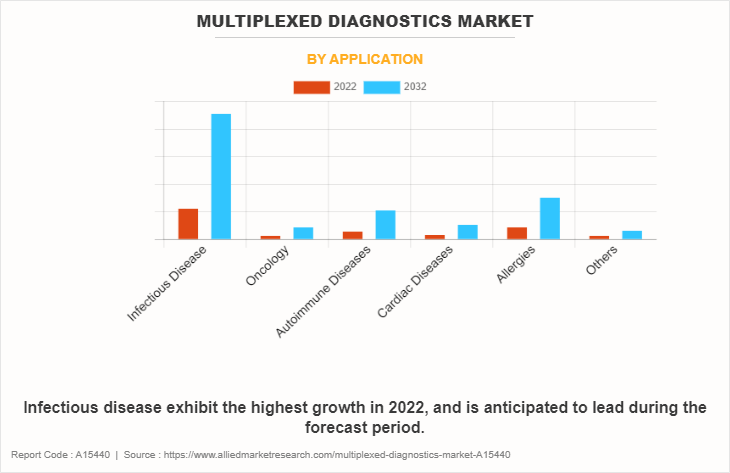
By Application
The multiplexed diagnostics market is segregated into infectious disease, oncology, autoimmune disease, cardiac disease, allergies, and others. The infectious disease segment dominated the market in 2022, owing to rise in prevalence of infectious disease. However, the autoimmune disease segment is expected to witness a significant CAGR during the forecast period.
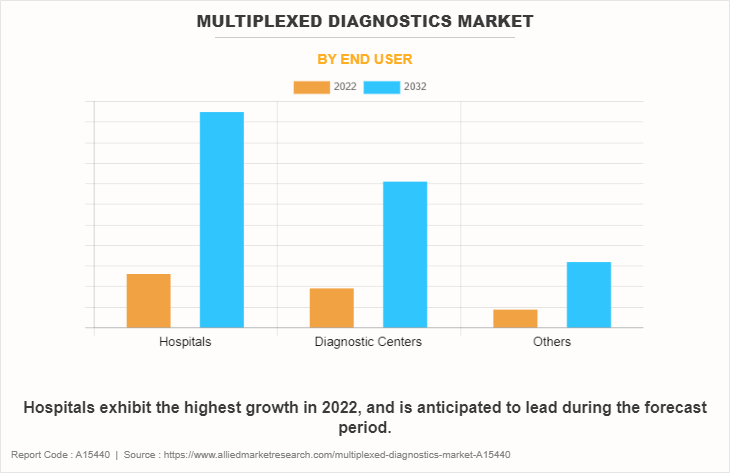
By End User
The multiplexed diagnostics market is segregated into hospitals, diagnostic centers, and others. The hospitals segment dominated the market in 2022, owing to significant applications of multiplexed diagnostics in hospitals such as it helps in simultaneous test for multiple genetic mutations or protein biomarkers associated with distinct types of cancer and for infectious disease testing and cardiovascular disease. However, the diagnostic centers segment is expected to witness a significant CAGR during the forecast period.
By Region
The multiplexed diagnostics market growth is studied across North America, Europe, Asia-Pacific, and LAMEA. North America accounted for a major share of the multiplexed diagnostics market in 2022 and is expected to maintain its dominance during the forecast period. This is attributed to the presence of key players, increase in and the rise in prevalence of chronic, infectious, and respiratory health issues. Moreover, the growing R&D activities in multiplexed diagnostics play a crucial role in biomarker discovery, aiding in the identification of novel biomarkers associated with diseases. For instance, PerkinElmer offers a range of diagnostic and research solutions, including multiplexed assays for biomarker discovery and validation, which has further contributed to the regional market growth.
Asia-Pacific is expected to grow at the highest rate during the multiplexed diagnostics market forecast due to a significant rise in healthcare IT spending and expanding healthcare infrastructure of developing countries such as China, India, and Japan. In addition, rise in the prevalence of chronic and infectious disease and increased R&D activities in developing countries are expected to drive the growth of the multiplexed diagnostics market in the region in the coming years.
Competition Analysis
Competitive analysis and profiles of the major players in the multiplexed diagnostics industry such as Co-Diagnostics, Inc. Agilent Technologies, Inc., bioMérieux SA, Illumina Inc., Hologic, Inc. Thermo Fisher Scientific Inc., Bio-Rad Laboratories Inc., Siemens Healthineers AG, F. Hoffmann-La Roche Ltd., and DiaSorin S.p.A. are provided in the report.
The key players in the multiplexed diagnostics industrysuch as bioMérieux SA, Hologic, Inc. F. Hoffmann-La Roche Ltd., Agilent Technologies Inc, Bio-Rad Laboratories and DiaSorin S.p.A have adopted product launch, product approval, acquisition, and partnership as major developmental strategies to improve the product portfolio.
Recent Product Approval in the Multiplexed Diagnostics Market
In May 2023, bioMérieux, a world leader in the field of in vitro diagnostics, had received the U.S. Food and Drug Administration (FDA) Clinical Laboratory Improvement Amendments (CLIA) waiver for the fast and accurate multiplex PCR-based BIOFIRE SPOTFIRE Respiratory (R) Panel Mini.
Recent Product Launch in the Multiplexed Diagnostics Market
In October 2021, Hologic, Inc had announced its broad European launch of the Novodiag system, a fully automated molecular diagnostic solution for on-demand testing of infectious diseases and antimicrobial resistance. The launch follows acquisition of Mobidiag Oy by Hologic in June 2021 and is projected to bring the benefits of the Novodiag system to a broader range of customers in Europe.
In August 2022, Roche announced the launch of the Digital LightCycler System, the first digital polymerase chain reaction (PCR) system of Roche. The Digital LightCycler System allows clinical researchers to divide DNA (deoxyribonucleic acid) and RNA from an already extracted clinical sample into as many as 100,000 microscopic individual reactions. The system can then perform PCR and produce highly sophisticated data analysis on the results.
Recent Partnership in the Multiplexed Diagnostics Market
In January 2023, Agilent Technologies, Inc. had announced a partnership with Akoya Biosciences, Inc. The Spatial Biology Company, to develop multiplex-immunohistochemistry diagnostic solutions for tissue analysis and to commercialize workflow solutions for multiplex assays in the clinical research market.
In June 2021, Bio-Rad Laboratories, Inc. a global leader of life science research and clinical diagnostic products, had announced a partnership with Seegene, Inc., a global leader in multiplex molecular diagnostics, for the clinical development and commercialization of infectious disease molecular diagnostic products.
Recent Acquisition in the Multiplexed Diagnostics Market
In July 2021, DiaSorin S.p.A. announced its acquisition of Luminex Corporation. Through the acquisition, DiaSorin gain access to Luminex’s multiplexing technology and a portfolio that strengthen its existing offering, while expanding the Group presence in the United States.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the multiplexed diagnostics market analysis from 2022 to 2032 to identify the prevailing multiplexed diagnostics market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the multiplexed diagnostics market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global multiplexed diagnostics market trends, key players, market segments, application areas, and market growth strategies.
Multiplexed Diagnostics Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 41.5 billion |
| Growth Rate | CAGR of 14.5% |
| Forecast period | 2022 - 2032 |
| Report Pages | 425 |
| By Product Type |
|
| By Application |
|
| By End User |
|
| By Region |
|
| Key Market Players | Co-Diagnostics, Inc., Bio-Rad Laboratories Inc, Illumina Inc., Agilent Technologies, Inc., BioMerieux SA, Siemens Healthineers AG, F. Hoffmann-La Roche Ltd., Hologic Inc., Thermo Fisher Scientific Inc., Diasorin S.p.A. |
Analyst Review
The multiplex detection of relevant biomarkers not only provides insight into the pathophysiology of cardiovascular disease, but also provides a guide for the most efficient treatment option. Most cancers have biomarkers in common with other cancers, hence detecting multiple biomarkers is needed for the accurate differentiation of cancer types or location.
The multiplexed detection of these biomarkers enables oncologists to accurately diagnose the patients and select the appropriate therapy, thus improving patient outcomes and decreasing healthcare costs. The rise in incidence of chronic diseases including cancer, tuberculosis, and other bacterial infections has increased the need for efficient and accurate diagnostic tools to manage these conditions and the advantages offered by multiplexed diagnostics such as it is more efficient and time savings as compared to traditional diagnostic tools and is cost-effective as compared to running multiple single-analyte assays are the factors that are anticipated to boost the growth of the market.
Moreover, increase in launches and approvals of new and advanced multiplexed diagnostic tools, are expected to fuel growth of the multiplexed diagnostic market. For instance, in February 2020, Co-Diagnostics, Inc., received the CE mark for Logix Smart Coronavirus COVID-19 Test from the European Community. The Logix Smart Coronavirus COVID-19 test kit is an in vitro diagnostic test, that operates using the real-time reverse transcriptase polymerase chain reaction (RT-PCR) process along with enhanced multiplexing for identifying the coronavirus.
In addition, in January 2023, Agilent Technologies, Inc. had announced a partnership with Akoya Biosciences, Inc. the Spatial Biology Company, to develop multiplex-immunohistochemistry diagnostic solutions for tissue analysis and to commercialize workflow solutions for multiplex assays in the clinical research market.
Multiplexing is the process of simultaneously detecting or identifying multiple biomarkers in a single diagnostic test, which can be valuable for several different types of diseases.
Increase in prevalence of chronic disease, technological advancements in multiplexed diagnostics products and surge in demand for personalized medicine
Top companies such as Co-Diagnostics, Inc. Agilent Technologies, Inc., bioMérieux SA, Illumina Inc., Hologic, Inc. Thermo Fisher Scientific Inc., Bio-Rad Laboratories Inc., Siemens Healthineers AG, F. Hoffmann-La Roche Ltd., and DiaSorin S.p.A held a high market position in 2022.
Kits & reagents segment dominated the global market in 2022, and expected to continue this trend throughout the forecast period.
The global multiplexed diagnostics market was valued at $10.7 billion in 2022, and is projected to reach $41.5 billion by 2032, growing at a CAGR of 14.5% from 2023 to 2032.
Expansion of applications of multiplexed diagnostics and increase in demand for personalized medicine are the major factors that are expected to create lucrative opportunities for the growth of the market.
North America is anticipated to witness lucrative growth during the forecast period, owing to presence of advanced healthcare infrastructure, increasing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular diseases
The forecast period for multiplexed diagnostics market is 2023 to 2032
Loading Table Of Content...
Loading Research Methodology...



