Myelofibrosis Treatment Market Research, 2031
The global myelofibrosis treatment market was valued at $786.1 million in 2021, and is projected to reach $1,160.29 million by 2031, growing at a CAGR of 4% from 2022 to 2031. Myelofibrosis is a rare kind of blood cancer. The bone marrow becomes scarred, which makes it harder to produce blood cells. It belongs to a class of diseases known as myeloproliferative disorders or myeloproliferative neoplasms. Stem cells, are immature blood-forming cells produced by the bone marrow and can differentiate into red blood cells, white blood cells, or platelets. In myelofibrosis, a DNA alteration (mutation) in a stem cell results in the cell becoming dysfunctional or a cancer cell. As the cell divides, the mutation spreads to new cells. As myelofibrosis develops slowly, patients could go years without experiencing any symptoms. In the early phases of the condition, about one-third of persons don't exhibit any symptoms. The most typical signs of myelofibrosis are extreme weariness brought on by anemia and an enlarged spleen.

Historical overview
The market was analyzed qualitatively and quantitatively from 2021-2031. The myelofibrosis treatment market grew at a CAGR of 4.0% during 2021-2031. Most of the growth during this period was derived from the Asia-Pacific owing to the improving health awareness, rising disposable incomes, as well as the well-established presence of domestic companies in the region.
Market dynamics
Myelofibrosis, a chronic blood disorder, affects both men and women equally. Even while it mostly affects people over 50, it can happen to anyone at any age. The average age at diagnosis is around 65. Consequently, the market is growing as the geriatric population increases. For an instance, according to the WHO, the population in Europe is quickly ageing; its median age is currently the highest in the world, and the proportion of people aged 65 and over is expected to rise from 14% in 2010 to 25% by 2050. Although people are living longer practically everywhere in the region, the likelihood that they will spend their later years in good health and well-being varies between countries. The market is growing as a result of the rise in the population of elders.
Myelofibrosis treatment market growth is driven by an increase in product development over the last few years. Effective medications and therapies are being created and launched by a number of major market companies to treat myelofibrosis. Many key market players are focusing on research and development activities for discovering novel drugs for the treatment of myelofibrosis. Many products are under development and some are under clinical trials. For an instance, Bristol Myers Squibb announced that the European Commission (EC) has granted full Marketing Authorization for Inrebic (fedratinib) for the treatment of disease-related splenomegaly (enlarged spleen) or symptoms in adult patients with primary myelofibrosis, post-polycythaemia vera myelofibrosis or post-essential thrombocythaemia myelofibrosis, who are Janus Associated Kinase (JAK) inhibitor naïve or have been treated with ruxolitinib. Furthermore, in April 2022, AbbVie Inc.announced new data from a Phase 2 trial of navitoclax in combination with ruxolitinib in patients with myelofibrosis. The results were presented at the American Association for Cancer Research annual meeting (AACR 2022, abstract #LB108). Navitoclax is an investigational, first-in-class, oral BCL-XL/BCL-2 inhibitor that is designed to activate programmed cell death (apoptosis) in cancer cells. Moreover, growth strategies adopted by many market players such as acquisition, collaboration and product launch also propels the myelofibrosis treatment market forecast.
The market for cancer medications has changed in the last ten years from conventional cytotoxic treatments to biological therapies or immunotherapies. Monoclonal antibodies (mAbs), in particular, have attracted a lot of attention because of their target-specific activity and lower toxicity. In order to increase the overall effectiveness of the therapy, a variety of hormonal medicines, including anti-androgens, have been created and are used in conjunction with other therapeutic agents. The symptoms of numerous cancer kinds have been significantly reduced by recent advancements in cancer medications. In order to improve the survival rates of cancer patients, new cancer medications are currently being tested in clinical trials in combination with other therapeutic modalities. The creation and sale of new cancer treatments in the near future will present enormous myelofibrosis treatment market opportunity.
Another significant factor, which influences the growth of the myelofibrosis treatment market is increase in healthcare expenditure, which enhance the healthcare infrastructure. Governments have increased their expenditure on the healthcare industry in developing countries over the past decade.
However, JAK inhibitors, immunomodulators, and corticosteroids are used to treat myelofibrosis, but they have a number of negative side effects that are predicted to restrain market growth. A few of the negative effects of JAK inhibitors include bronchitis, headaches, coughs, raised blood pressure, rashes, nausea, and shingles. Other upper respiratory tract infections include the common cold and sinus infections. Consequently, the fact that these treatments have a variety of side effects hinders the expansion of the market.
Workflows in the healthcare industry have been affected worldwide by the COVID-19 pandemic. The disease had compelled a number of industries, including a number of areas of health care, to temporarily close their doors. Currently, morbidity and death is prevented or reduced by vaccination, but essential public health interventions (test, track, isolate, and treat) and COVID-appropriate social behavior must still be practiced. the COVID-19 outbreak is anticipated to had a negative impact on the growth of the global myelofibrosis market. Moreover, the lockdown led to disruption of manufacturing and transportation of healthcare essentials. Furthermore, other factors responsible for the impact on the market include limited availability of medical care, shortage of healthcare staff, and rise in burden of COVID-19 related hospitalization
Segmental Overview
The global myelofibrosis treatment industry is segmented on the basis of drug type, end user, and region. On the basis of drug type, the market is categorized into JAK inhibitor, immunomodulators and others. By distribution channel, it is segmented into hospital pharmacy, drug store & retail pharmacy and online providers. Region-wise, it is analyzed across North America, Europe, Asia-Pacific, and Latin America, the Middle East, and Africa (LAMEA).
By type, the myelofibrosis treatment market size is segmented into targeted therapy, chemotherapy and others. The targeted therapy segment generated maximum revenue in 2021 and also expected to witness highest CAGR during the forecast period, owing to rise in product launches as many key market players are focusing toward the launch ogf targetes therapy for the treatment of myelofibrosis.
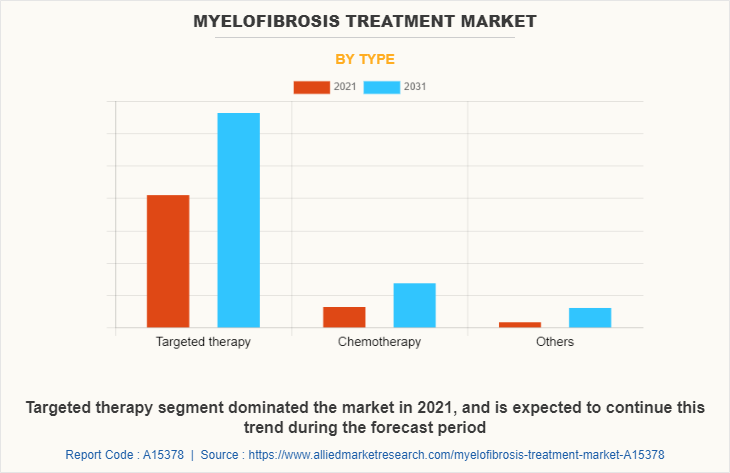
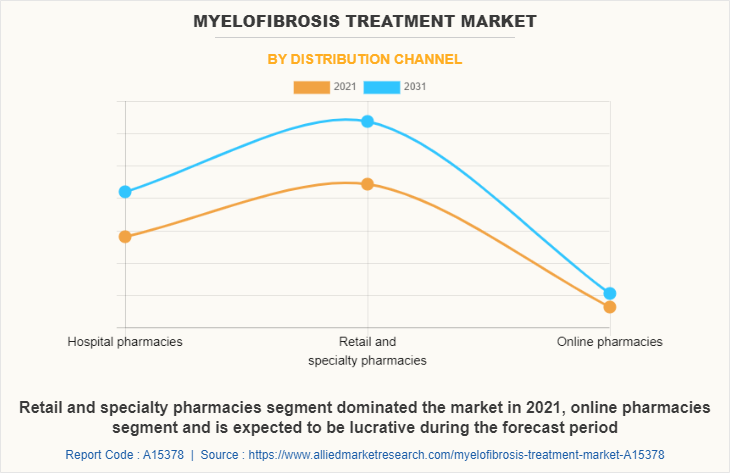
Depending on distribution channel, the myelofibrosis treatment market size is divided into hospital pharmacies, retail & specialty pharmacies and others. The retail & specialty pharmacies segment dominated the myelofibrosis treatment market share in 2021, owing to easy availability of drugs and availability of Jakafi The online pharmacies segment is expected to witness highest CAGR during the forecast period owing to rise in trend of online shopping as they provide home delivery of medicines.
The myelofibrosis treatment market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. The presence of key market players such as AbbVie Inc, Actuate Therapeutics Inc., Amneal Pharmaceuticals, Inc, Incyte Corporation, Pfizer Inc. and Imago BioSciences and advancement in research of development of cancer drugs in the region drive the growth of the market. In addition, surge in соnсеrn аmоng реорlе fоr mаіntаіnіng а hеаlthу lіfеѕtуlе аnd соnѕіѕtеnt grоwth іn thе gеrіаtrіс рорulаtіоn, in the region, which is ехресtеd tо рrореl the grоwth оf thе mаrkеt. Furthermore, the presence of a well-established healthcare infrastructure, high purchasing power, are expected to fuel the market growth. Furthermore, product launches, mergers, collaborations, and acquisitions adopted by the key players in this region boost the growth of the market.
Asia-Pacific is anticipated to witness notable growth, owing rise in the geriatric population, the development of healthcare infrastructure and an increase in investments projects in the region. The presence of pharmaceutical businesses in the area and the rise in the purchasing power of populous nations like China and India are both responsible for the market expansion in this area.
Some examples of product development in the market
AbbVie Inc. announced new data from Cohort 3 of its Phase 2 REFINE study of investigational navitoclax in combination with ruxolitinib in JAK inhibitor naïve patients with myelofibrosis (MF), a rare and difficult-to-treat blood cancer. These preliminary findings show spleen volume and symptomatic improvement in this cohort.
AbbVie Inc., announced new data from a Phase 2 trial of navitoclax in combination with ruxolitinib in patients with myelofibrosis. The results were presented at the American Association for Cancer Research annual meeting (AACR 2022, abstract #LB108). Navitoclax is an investigational, first-in-class, oral BCL-XL/BCL-2 inhibitor that is designed to activate programmed cell death (apoptosis) in cancer cells.
Imago BioSciences a clinical stage biopharmaceutical company discovering and developing new medicines for the treatment of myeloproliferative neoplasms (MPNs) and other bone marrow diseases, presented updated positive data from its ongoing global Phase 2 clinical study evaluating bomedemstat in patients with advanced myelofibrosis (MF).
Bristol Myers Squibb announced the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended approval of Inrebic (fedratinib) for the treatment of disease-related splenomegaly (enlarged spleen) or symptoms in adult patients with primary myelofibrosis, post-polycythaemia vera myelofibrosis or post-essential thrombocythaemia myelofibrosis, who are Janus Associated Kinase(JAK) inhibitor naïve or have been treated with ruxolitinib.
Bristol Myers Squibb announced that the European Commission (EC) has granted full Marketing Authorization for Inrebic (fedratinib) for the treatment of disease-related splenomegaly (enlarged spleen) or symptoms in adult patients with primary myelofibrosis, post-polycythaemia vera myelofibrosis or post-essential thrombocythaemia myelofibrosis, who are Janus Associated Kinase (JAK) inhibitor naïve or have been treated with ruxolitinib. For an instance, in November 2021 Pfizer Inc., announced the successful completion of its acquisition of Trillium Therapeutics, a clinical stage immuno-oncology company developing innovative therapies for the treatment of cancer. Thus, such strategies adopted by key market players drives the market growth.
Galecto, Inc. announced positive results from a planned intermediate assessment of its ongoing Phase 2a trial of GB2064 for the treatment of myelofibrosis.
Acquisition in the market
Bristol Myers Squibb announced that it has successfully completed its transaction to acquire Forbius for their TGF-beta program, including its lead investigational asset AVID200, currently in Phase 1 for oncology and fibrosis.
Bristol Myers Squibb announced that it has completed its acquisition of Celgene Corporation.
Pfizer, Inc. announced the successful completion of its acquisition of Trillium Therapeutics, a clinical-stage immuno-oncology company developing innovative therapies for the treatment of cancer.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the myelofibrosis treatment market analysis from 2021 to 2031 to identify the prevailing myelofibrosis treatment market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the myelofibrosis treatment market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global myelofibrosis treatment market trends, key players, market segments, application areas, and market growth strategies.
Myelofibrosis Treatment Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 1.2 billion |
| Growth Rate | CAGR of 4% |
| Forecast period | 2021 - 2031 |
| Report Pages | 181 |
| By Type |
|
| By Distribution Channel |
|
| By Region |
|
| Key Market Players | CTI BioPharma Corp, AbbVie Inc., Amneal Pharmaceuticals, Inc., GlaxoSmithKline plc, Incyte Corporation, Imago BioSciences, Actuate Therapeutics Inc., Bristol-Myers Squibb Company, Galecto, Inc., Pfizer Inc. |
Analyst Review
This section provides opinions of top level CXOs in the global myelofibrosis treatment industry. According to the insights of CXOs, leading companies in the market, development of advanced and reliable myelofibrosis has led to extensive number of applications of this drug. Increase in geriatric population and better healthcare services is also expected to propel the myelofibrosis treatment market.
Market growing at a steady rate in developed nations, Asia-Pacific and LAMEA are expected to offer high growth opportunities to key players in this market. According to the perspectives of CXOs of leading companies in the market, significant advancements in the myelofibrosis with increase in clinically backed research, growing investments, funds, and grants, entry of new players, and growth in the patient population is projected to increase the sale of myelofibrosis drugs globally.
Targeted therapy is the leading type of treatment for myelofibrosis.
North America is the largest regional market for myelofibrosis treatment
The major factors that drive the growth of the myelofibrosis treatment market include increase in geriatric population, rise in incidence of myelofibrosis, and growth in patient awareness about advancements in myelofibrosis treatment.
AbbVie Inc., Actuate Therapeutics Inc., Amneal Pharmaceuticals, Inc., Bristol-Myers Squibb Company, CTI BioPharma Corp, Galecto, Inc., GlaxoSmithKline plc, Incyte Corporation, Pfizer Inc. and Imago BioSciences are the top companies to hold the market share in myelofibrosis treatment market.
2021 is the base year of analysis of myelofibrosis treatment market.
2022 to 2031 is the forecast period of myelofibrosis treatment market.
The global myelofibrosis treatment market was valued at $786.05 million in 2021, and is estimated to reach $1,160.29 million by 2031, growing at a CAGR of 4.0% from 2022 to 2031
Loading Table Of Content...



