Pediatric Neurology Device Market Research, 2031
The global pediatric neurology device market was valued at $2.8 billion in 2021, and is projected to reach $5.8 billion by 2031, growing at a CAGR of 7.8% from 2022 to 2031. Pediatric neurology is a specialized branch of medicine that deals with the diagnosis and management of neurological conditions in neonates (newborns), infants, children, and adolescents. Neurological disorders have a wide spectrum and can have various causes, complications, symptoms, and results.

The child neurology includes diseases and disorders of the spinal cord, brain, peripheral nervous system (PNS), autonomic nervous system (ANS), muscles, and blood vessels that affect individuals in these age groups. The disease conditions require various devices for the purpose of diagnosis and treatment procedures. Some of the devices used in pediatric patients include the neurosurgical devices used in surgical treatment procedures or implant devices for regular management of the brain CSF.
Market Dynamics
The growth in the diagnostic and biotechnology industry for the adoption of pediatric neurological devices is expected to increase at a faster rate owing to the massive pool of neurological diseases among the target population, which creates an opportunity for the Pediatric Neurology Device Industry. Rise in launch of new tests due to advanced technology by various key players across the globe is set to affect the market growth positively. For instance, in February 2019, Medtronic plc announced both the U.S. launch of Deep Brain Stimulation (DBS) for medically refractory epilepsy and the first commercially implanted patient at Emory University in Atlanta, Ga. DBS therapy for epilepsy delivers controlled electrical pulses to a target in the brain called the anterior nucleus of the thalamus (ANT), which is part of a network involved in seizures. The growth of the pediatric neurology devices market is also expected to be driven by high potential in adoption of the devices in developing countries due to increase in the prevalence of neurological disorders, increase in practice to diagnose the children for neurological diseases, and surge in demand for pediatric neurological devices.
Furthermore, the healthcare industry in emerging economies is developing at a significant rate, owing to significant investments by government to improve pediatric healthcare, and strong foothold of medical technology industries in emerging countries. In addition, the launch of cost-effective devices may also decide the adoption of the device. For instance, Medtronic plc announced the launch of the Solitaire (TM) X Revascularization Device in the U.S. and its first use in patients for the treatment of acute ischemic stroke. In addition, Medtronic introduced Phenom (TM) 21 Catheter, a 160cm length microcatheter for the delivery of all sizes of Solitaire X. The Solitaire X device mechanically removes blood clots from blocked vessels in the brain to restore blood flow in patients suffering an acute ischemic stroke with large vessel occlusion. Solitaire X provides a low clot crossing profile, with smooth delivery to occlusions in distal vessels as small as 2.0mm with the combination of device with Phenom 21. Hence, the product launch factor for pediatric neurology devices is attributed to further support the Pediatric Neurology Device Market Growth. The demand for pediatric neurology devices is not only limited to developed countries, but is also being witnessed in the developing countries, such as China, Brazil, and India, which fuel the growth of the market. Factors such as rise in adoption of neurological devices among the pediatric patients and increase in awareness toward chronic neurological diseases risks further drive the growth of the market. Furthermore, rise in consumer awareness related to preventive healthcare of newborns boosts the adoption of pediatric neurology devices. Moreover, an increase in research activities by manufacturers for developing innovative solutions in pediatric neurology devices technology is expected to fuel their adoption in the near future.
However, the cost of neurological disease treatment is hampering the market growth. Moreover, stringency in product approval is anticipated to hamper the growth of the market. Increasing R&D activities in the field of medical devices along with significant improvement in medical infrastructure are anticipated to provide new opportunities for the expansion of the market.
The pediatric neurology device market is segmented into Product Type, Condition, Age group and End User.
The pediatric neurology devices market is segmented on the basis of product type, condition, age group, end user, and region. On the basis of product, the market is categorized into neurostimulator devices, neurosurgery devices, interventional neurology devices, cerebrospinal fluid (CSF) management devices and others. On the basis of condition, the market is classified into stroke, seizures, hydrocephalus, oncology and others. Based on age group, the market is classified into 0-5 years, 5-10 years and 10-15 years. On the basis of end user, the market is classified into hospitals, ambulatory surgery centers and others. Region wise, the market is studied across North America (the U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
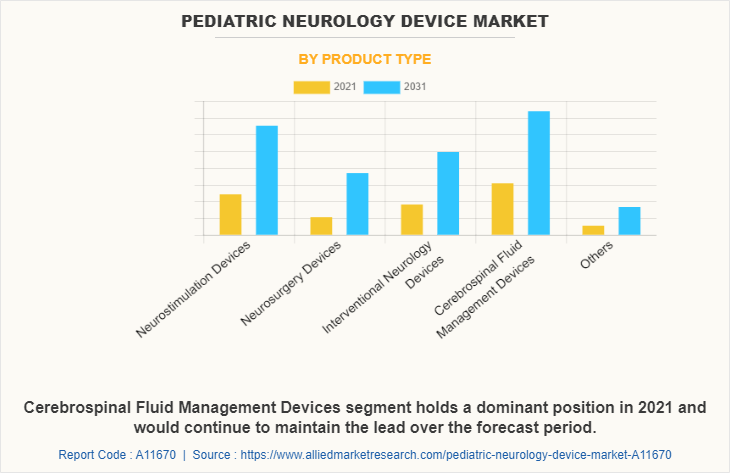
By Product Type
The cerebrospinal fluid management devices segment dominated the global Pediatric Neurology Device Market Share in 2021 and is expected to remain dominant throughout the forecast period, owing to the increase in the adoption of cerebrospinal fluid management devices for surgical procedures of the patients and availability of several types of devices developed by various key players in the market.
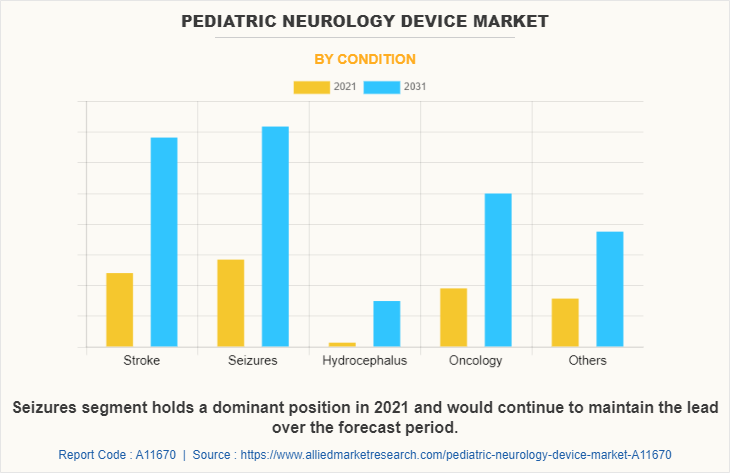
By Condition
The seizures segment dominated the global Pediatric Neurology Device Market Size in 2021 and is anticipated to continue this trend during the forecast period. This is attributed to the increase in the prevalence of stroke among the pediatric population. In addition, the launch of new devices and an increase in the support from the government authorities for the cost-effective treatment of pediatric patients are contributes to the growth of the market.
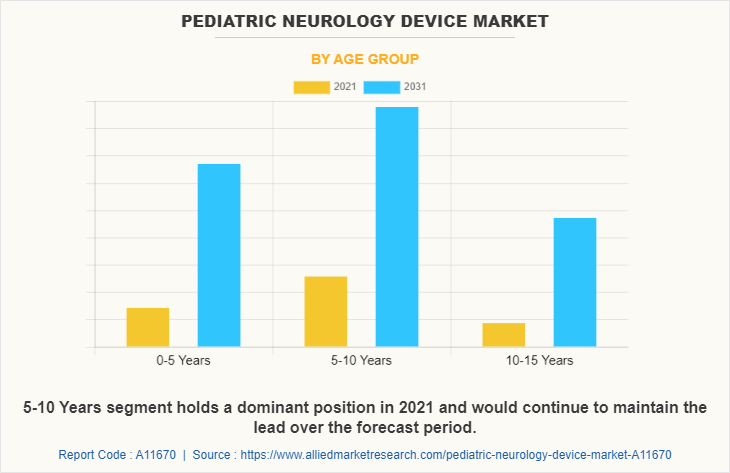
By Age Group
The 5-10 years segment dominated the global Pediatric Neurology Device Market Size in 2021 and is anticipated to continue this trend during the forecast period. This is attributed to the increase in the prevalence of neurological diseases among the target population. In addition, growth in awareness regarding neurological conditions drives the growth of the market.
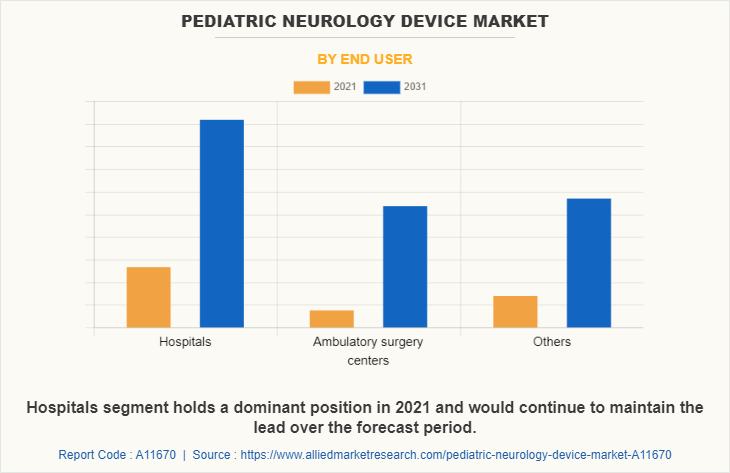
By End User
The hospitals segment held the largest market share in 2021 and is expected to remain dominant throughout the Pediatric Neurology Device Market Forecast period, owing to the preference of the population for the treatment of diseases among the hospitals and ease of the accessibility of the hospital's admissions post-COVID-19 pandemic situation.
By Region
In 2021, North America was the dominant region and is expected to remain dominant throughout the forecast period, owing to high prevalence rate of neurological disease such as seizures and hydrocephalus among pediatric population and increase in the number of market players available in the region. However, Asia-Pacific is expected to witness the highest CAGR during the analysis period, owing to the presence of high populace countries such as India and China, which in turn increases the number of chances of suffering from genetic conditions, and the increasing number of strategies and trends adopted by the market players.
COMPETITION ANALYSIS
Competitive analysis and profiles of the major players in the pediatric neurology devices, such as Abbott Laboratories, B. Braun Melsungen AG, Boston Scientific Corporation, Integra LifeSciences Holdings KARL STORZ SE & Co. KG., LivaNova PLC, Medtronic plc, Natus Medical Incorporated (ArchiMed), Stryker Corporation, Zimmer Biomet are provided in this report. There are some important players in the market such as Abbott Laboratories, Boston Scientific Corporation, LivaNova PLC and Medtronic plc. Various players have adopted strategies such as product launch and product approval as key developmental strategies to improve the product portfolio of the pediatric neurology devices market.
Product launch in the market
In January 2021, Medtronic plc announced the launch of ADAPT-PD (Adaptive DBS Algorithm for Personalized Therapy in Parkinson's Disease). Adaptive deep brain stimulation is an investigational feature of the Percept PC device that could be enabled if approved.
In October 2019, Medtronic plc announced the U.S. launch of its advanced Patient Programmer technology for Deep Brain Stimulation (DBS) therapy at the Samsung Developers Conference in San Jose, Calif. The new programmer which received U.S. Food and Drug Administration (FDA) approval on July 3, 2019, leveraged a user-friendly, custom-configured Samsung mobile device, which allows patients to manage their therapy more simply and discreetly.
Product approval in the market
In July 2021, Medtronic plc announced the U.S. Food and Drug Administration (FDA) approval and first U.S. implants of the SenSight Directional Lead System used for Deep Brain Stimulation (DBS) therapy. SenSight was the first-of-its-kind DBS directional lead that combines the benefits of directionality with the power of sensing, allowing physicians to deliver precise, patient-specific DBS therapy for the treatment of some symptoms associated with movement disorders such as Parkinson's disease, dystonia and essential tremor, and medically refractory epilepsy.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the pediatric neurology device market analysis from 2021 to 2031 to identify the prevailing Pediatric Neurology Device Market Opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the pediatric neurology device market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global pediatric neurology device market trends, key players, market segments, application areas, and market growth strategies.
Pediatric Neurology Device Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 5.8 billion |
| Growth Rate | CAGR of 7.8% |
| Forecast period | 2021 - 2031 |
| Report Pages | 220 |
| By Product Type |
|
| By Condition |
|
| By Age group |
|
| By End User |
|
| By Region |
|
| Key Market Players | Boston Scientific Corporation, Abbott Laboratories, Zimmer Biomet Holding Inc., Natus Medical Incorporated, B.Braun SE, LivaNova PLC, Integra LifeSciences Holdings, Medtronic plc, Stryker Corporation, KARL STORZ SE & Co. KG |
Analyst Review
In accordance to several interviews conducted, the process of diagnosing and treating a child with a neurological condition may be stressful task. Neurological disorders in children occur owing to abnormality in the brain, the nervous system or the muscle cells. These disorders vary from epilepsy to migraine headaches to tic or movement disorders and many more. The pediatric population may be born with the disorder, such as spina bifida or hydrocephalus, or they may acquire the disorder later in life. The medical devices approved by the regulating bodies such as U.S. Food and Drug Administration (FDA) may help in the treatment and proper management of the neurological diseases in pediatric patients.
Asia-Pacific is expected to witness the highest CAGR during the analysis period, owing to the increase in the research and development expenditure for the development of the new products that increases the adoption rate of pediatric neurology device, increase in awareness about the neurological diseases among the adolescents and newborn, increase in the number of hospital admissions of the pediatric patients and increase in government support on research activities and other related trends adopted by the market players
The total market value of the Pediatric Neurology Device Market is $2764.9 million in 2021.
The forecast period in the report is from 2022 to 2031.
North America the largest regional market for Pediatric Neurology Device
The market value of the Pediatric Neurology Device Market in 2022 was $2969.5 million.
The top companies to hold the market share in Pediatric Neurology Device are Abbott Laboratories, B. Braun Melsungen AG, Boston Scientific Corporation, Integra LifeSciences Holdings KARL STORZ SE & Co. KG., LivaNova PLC, Medtronic plc, Natus Medical Incorporated (ArchiMed), Stryker Corporation, Zimmer Biomet.
The base year for the report is 2021.
Yes, Pediatric Neurology Device companies are profiled in the report.
The key trends in the Pediatric Neurology Device Market are increasing incidence of hydrocephalus in children, and a surge in technological advancement in Pediatric Neurology Devices.
Loading Table Of Content...



