Bispecific Antibody Market Research, 2032
The global bispecific antibody market size was valued at $5.5 billion in 2022, and is projected to reach $109.4 billion by 2032, growing at a CAGR of 34.8% from 2023 to 2032. A bispecific antibody is a type of artificial antibody designed to simultaneously bind to two different target molecules. Bispecific monoclonal antibody engage two distinct antigens, cells, or other molecules with high affinity. This unique feature allows them to bring these targets together, enabling a variety of therapeutic applications. Bispecific antibodies have applications in the field of immunotherapy and targeted therapy for various diseases, including cancer, autoimmune disorders, and infectious diseases. Bispecific monoclonal antibody enhance the specificity and efficacy of therapeutic interventions by simultaneously targeting multiple molecules. Their versatility and potential applications have made them a subject of significant R&D in the biomedical and pharmaceutical industries.

Market Dynamics
Growth of the global bispecific antibody market share is majorly driven by high presence of market players bispecific antibody, increase in number of product approvals for bispecific antibody, and technological advancements for development of bispecific antibody. Moreover, rise in prevalence of chronic diseases such as cancer, heart disease, stroke, diabetes, and arthritis are anticipated to increase the need for bispecific antibodies, and boost the growth of the bispecific antibody market. Bispecific antibodies treat cancer by engaging the tumor-specific antigen CD20 and the CD3 antigen on T cells. Thus, rise in prevalence of cancer drives market growth. For instance, according to the American Cancer Society, in 2022, 1,918,030 new cancer cases and 609,360 cancer deaths were projected to occur in the U.S., including approximately 350 deaths per day from lung cancer, the leading cause of cancer death.
Furthermore, according to the same source, in 2022, around 288,300 new instances of prostate cancer were discovered, with 34,700 deaths caused due to disease. In addition, rise in the number of metastatic urothelial carcinoma and increase in application of bispecific antibodies for treatment of metastatic urothelial carcinomas is anticipated to drive the growth of the market. For instance, in June 2023, the Food and Drug Administration (FDA), accelerated the approval for the combination of (enfortumab vedotin-ejfv) Padcev and pembrolizumab marking an important milestone for the approximately 8000 to 9000 patients in the U.S. with locally advanced or metastatic urothelial cancer who were not eligible for cisplatin-containing chemotherapy.
Bispecific monoclonal antibody commonly bind to a tumor epitope along with CD3 on T-cells, leading to T-cell activation and tumor cell killing. These treatments show great promise in multiple myeloma (MM) patients. Moreover, in accordance with Johnson & Johnson, a pharmaceutical company, in 2022, more than 34,000 people were estimated to be diagnosed with multiple myeloma, and more than 12,000 people to die from the disease in the U.S. Thus, rise in the prevalence of chronic diseases anticipated to increase the demand for bispecific antibody and fuel the growth of bispecific antibody market size.
In addition, rise in number of product approvals for bispecific antibodies is anticipated to drive the bispecific antibody market growth. For instance, in July 2023, AbbVie, American pharmaceutical company announced that the U.S. Food and Drug Administration (FDA) approved Epkinly (epcoritamab-bysp), as the first and only T-cell engaging bispecific antibody for the treatment of adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). In addition, in December 2022, Genentech announced the Food and Drug Administration (FDA) approval for Lunsumio, a first-in-class bispecific antibody, to treat people with relapsed or refractory follicular lymphoma.
Moreover, in August 2022, The Janssen Pharmaceutical Companies of Johnson & Johnson announced that the European Commission (EC) has granted conditional marketing authorisation (CMA) of TECVAYLI (teclistamab) as monotherapy for the treatment of adult patients with relapsed and refractory multiple myeloma (RRMM). It is a first-in-class bispecific antibody for the treatment of patients with multiple myeloma.
Moreover, increase in the number of R&D activities for development of bispecific antibodies is anticipated to fuel the bispecific antibody market opportunity. For instance, according to the Journal, eCancer, in August 2022, it was reported that Glioblastoma multiform (GBM) is one of the most aggressive, fast-growing tumors, and the most common brain cancer with a median overall survival of approximately 15 months. Novel T cell bispecific antibody shows potent anti-tumor activity in preclinical models of EGFRvIII mutant glioblastoma.
Moreover, rise in number of strategic collaborations for R&D is anticipated to boost the bispecific antibody market forecast. For instance, in January 2021, Loxo Oncology at Lilly, a R&D group of Eli Lilly and Company, and Merus N.V., a clinical-stage oncology company, announced a research collaboration and exclusive license agreement that is expected to leverage proprietary Biclonics platform of Merus along with the scientific and rational drug design expertise of Loxo Oncology at Lilly to research and develop up to three CD3-engaging T-cell re-directing bispecific antibody therapies.
On the other hand, side effects associated with bispecific antibodies treatment include cytokine release syndrome (CRS), immune effector cell associated neurotoxicity (ICANS), cytopenia, and infections that are expected to hamper the growth of the market. A potentially life-threatening complication in which activated immune cells release a large number of cytokines into the bloodstream, resulting in systemic inflammation.
For instance, a study published in Blood Cancer Journal, in March 2022, reported that patients treated with BsAb therapy for multiple myeloma experienced the side effects of treatment including 72% of patients experienced cytokine release syndrome (CRS) and 5% experienced immune effector cell-associated neurotoxicity syndrome (ICANS). Furthermore, 87% of patients experienced hypogammaglobulinemia, and 53% received immunoglobulin replacement. This factor leads to decrease in the treatment of bispecific antibodies, which is expected to hamper the growth of the bispecific antibody market size.
Segmental Overview
The bispecific antibody market share is segmented on the basis of product, application, end user, and region. On the basis of product, the market is categorized into blinatumomab, Emicizumab, and others. Others subsegment includes elranatamab, mosunetuzumab, glofitamab, and zenocutuzumab. On the basis of application, the market is divided into cancer, autoimmune diseases, and others. Others subsegment includes hemophilia, neovascular age-related macular degeneration, and Alzheimer's disease. On the basis of end user, the market is divided into hospitals, cancer center, and others. Others subsegment includes research centers and academic institutions.
On the basis of region, the bispecific antibodies market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and rest of Europe), Asia-Pacific (China, Japan, India, Australia, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, Saudi Arabia, South Africa, and rest of LAMEA).
Based Product
The bispecific antibody market is classified into blinatumomab, emicizumab, and others. Others includes elranatamab, mosunetuzumab, glofitamab, and zenocutuzumab. The emicizumab segment is expected to be the fastest growing segment during the forecast period owing to the rise in prevalence of hemophilia and increase in number of research activities for development of bispecific antibodies.
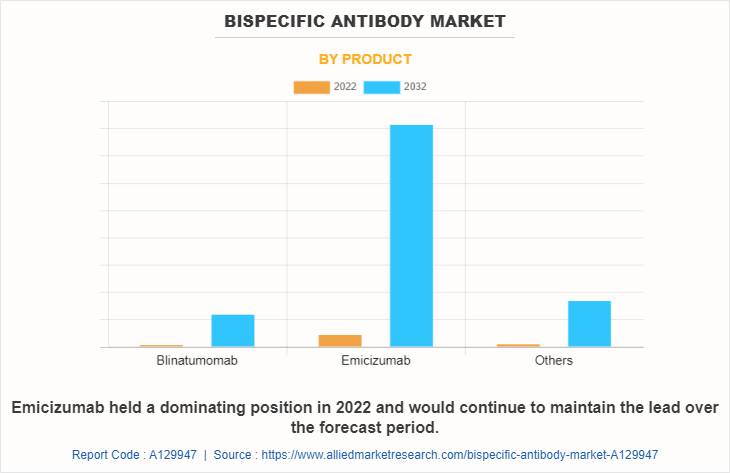
Based Application
The bispecific antibody market is classified into cancer, hemophilia, and others. Others includes autoimmune diseases, neovascular age-related macular degeneration and Alzheimer’s disease. The hemophilia segment is projected to be the fastest growing segment during the forecast period owing to a rise in the number of populations suffering from hemophilia and increase in awareness among the people regarding treatment of hemophilia.
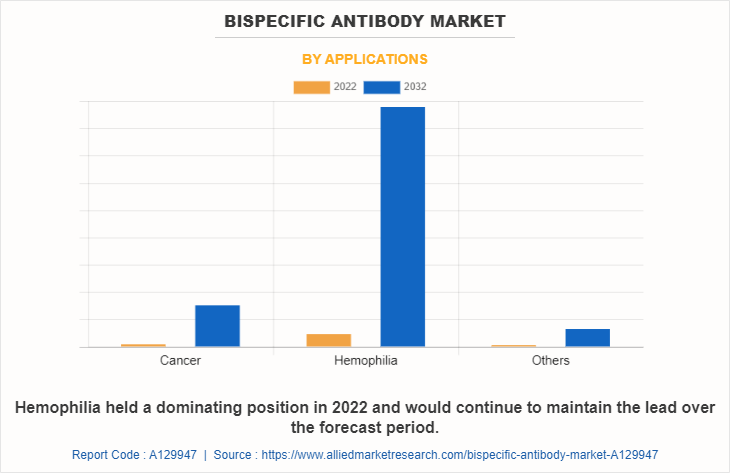
Based on End User
The bispecific antibody market is classified into hospitals, cancer center, and others. The hospital segment is projected to be the fastest growing segment during the forecast period owing to rise in the number of initiatives taken by government for development of healthcare infrastructure and rise in the prevalence of chronic diseases and autoimmune diseases.
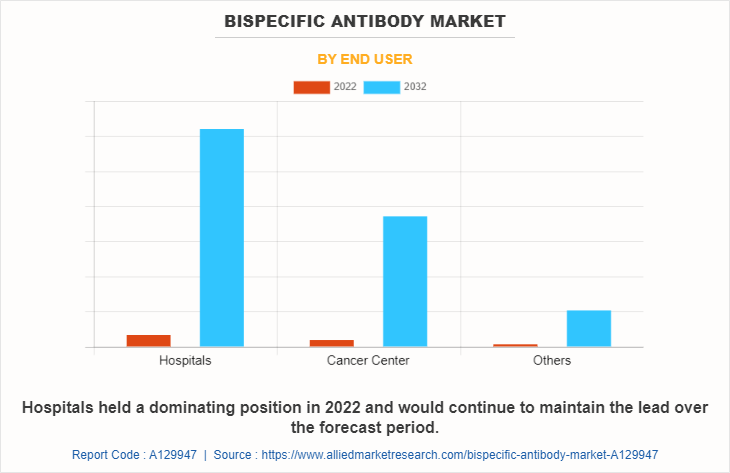
Based on Region
The North America bispecific antibody industry is expected to rise over the forecast period due to an increase in cancer prevalence caused by factors such as lifestyle changes, smoking, lower physical activity, and high presence of market players who manufacture bispecific antibody. For instance, according to National Center for Health Statistics, in January 2022, 1,918,030 new cancer cases and 609,360 cancer deaths were projected to occur in the U.S. Moreover, high presence of market players who manufacture bispecific antibody was anticipated to drive the growth of market. For instance, Janssen Pharmaceutical Companies of Johnson & Johnson, Amgen, AbbVie, Bristol Myers Squibb Company are major market players who manufacture bispecific antibodies.
Moreover, rise in number of product approvals of bispecific antibodies by U.S. companies is anticipated to drive the growth of market in North America. For instance, in July 2023, AbbVie, a pharmaceutical company announced that the U.S. Food and Drug Administration (FDA) approved Epkinly (epcoritamab-bysp), as the first and only T-cell engaging bispecific antibody for the treatment of adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL).
Moreover, rise in number of adoptions of key strategies collaboration, partnership and others by market players in the U.S. is expected to drive the growth of market. For instance, in May 2021, Bristol Myers Squibb, a pharmaceutical company, and Agenus Inc., a biotechnology research company announced that they have entered into a definitive agreement under which Bristol Myers Squibb will be granted a global exclusive license to Agenus’ proprietary bispecific antibody program, AGEN1777, that blocks TIGIT and a second undisclosed target. AGEN1777 is an Fc-enhanced antibody in late preclinical development designed to target major inhibitory receptors expressed on T and NK cells to improve anti-tumor activity. High presence of market players and rise in number of adoptions of key strategies collaboration, partnership and others by market players is thus expected to boost the growth of bispecific antibody market.
However, the Asia-Pacific bispecific antibody industry is expected to grow during the forecast period, owing to rise in prevalence of chronic disease such as cancer, autoimmune diseases, and others. This factor is anticipated to drive the demand for bispecific antibody for treatment purpose and drives the growth of market. For instance, according to National Library of Medicine, the estimated number of incident cases of cancer in India for the year 2022 were found to be 14,61,427. As per the same source, in India, one in nine people were expected to develop cancer in his/her lifetime. Lung and breast cancers were the leading sites of cancer in males and females, respectively.,
Moreover, increase in the strategic partnership between the market players is anticipated to boost the need for bispecific antibody treatment. Thus, driving the growth of the bispecific antibody market in Asia-Pacific. For instance, in September 2022, Abpro Bio Co., a biotechnology company, announced the strategic partnership with Celltrion, a global biopharmaceutical company. Celltrion granted an exclusive license to Abpro Bio to develop and commercialize two bispecific antibody candidates ABP-100 and ABP-201 of Abpro. ABP-100 is in development for immuno-oncology with initial indications including gastric, breast, and endometrial cancers. ABP-201 is in development for ophthalmology with initial indications inclusive of Wet AMD (age-related macular degeneration) and diabetic macular edema. Thus, strategic partnership among market players boosts the growth of the bispecific antibodies market in Asia-Pacific.
Competition Analysis
Some of the major companies that operate in the global bispecific antibody market include AbbVie, Pfizer, Portola Pharmaceuticals, Innovent biologics, Merus, MacroGenics Inc., Genmab A/S, AstraZeneca, NovImmune S.A, Regeneron Pharmaceuticals, F. Hoffmann-La Roche Ltd., Johnson & Johnson.
Recent Product Approval in the Bispecific Antibody Market
In March 2023, InvoX Pharma Limited, a biopharmaceutical company announced that F-star, an InvoX company, entered into a second license agreement with Takeda Pharmaceutical, a leading global, values-based, R&D-driven biopharmaceutical company for a novel next-generation immuno-oncology bispecific antibody.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the bispecific antibody market analysis from 2022 to 2032 to identify the prevailing bispecific antibody market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the bispecific antibody market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global bispecific antibodies market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the bispecific antibody market players.
- The report includes the analysis of the regional as well as global bispecific antibodies market trends, key players, market segments, application areas, and market growth strategies.
Bispecific Antibody Market Report Highlights
| Aspects | Details |
| Market Size By 2032 | USD 109.4 billion |
| Growth Rate | CAGR of 34.8% |
| Forecast period | 2022 - 2032 |
| Report Pages | 382 |
| By Applications |
|
| By End User |
|
| By Product |
|
| By Region |
|
| Key Market Players | Amgen Inc., AbbVie Inc, AstraZeneca plc, Regeneron Pharmaceuticals, Inc., Merus, Johnson & Johnson, Bristol-Myers Squibb Company, Pfizer Ltd, MacroGenics Inc., F. Hoffmann-La Roche Ltd. |
Analyst Review
Bispecific antibodies, constructed via quadroma, chemical conjugation, and genetic recombination, exert effector functions beyond natural antibodies by redirecting cells or modulating different pathways. It has multiple therapeutic applications and contributes to improved therapy responses in individuals with resistant tumors.
High presence of market players who manufacture bispecific antibodies and rise in the number of adoptions of key strategies such as partnership, agreement, product launch, and product approval are anticipated to boost the growth of the market. For instance, in April 2023, Lonza, a global pharmaceutical, biotech, and nutrition company announced an agreement with ABL Bio, the leading biotech research company of the world focusing on bispecific antibodies for immuno-oncology and neurodegenerative diseases. The collaboration is intended to help in the development and manufacturing of new bispecific antibody product of the company.
In addition, in March 2023, invoX Pharma Limited, a biopharmaceutical company announced that F-star, an invoX company, entered into a second license agreement with Takeda Pharmaceutical, a leading global, values-based, R&D-driven biopharmaceutical company for a novel next-generation immuno-oncology bispecific antibody.
Moreover, in January 2023, Caris Life Sciences, a molecular science company, and Xencor Inc., a clinical-stage biopharmaceutical company announced an expansion of their collaboration to research, develop and commercialize novel XmAb. Caris developed and presented XmAb bispecific and multi-specific antibodies directed against new targets for the treatment of cancer patients.
The top companies that hold the market share in bispecific antibody market are AbbVie, Pfizer, Portola Pharmaceuticals, Innovent biologics, Merus, MacroGenics Inc., and Johnson & Johnson.
North America is anticipated to witness lucrative growth during the forecast period, owing to rise in expenditure by government organization to develop the healthcare sector, increase in the prevalence of chronic diseases and increase in the number of geriatric populations.
The key trends in the bispecific antibody market are rise in number of populations suffering from hemophilia and increase in number of drug approval of bispecific antibody.
The base year for the report is 2022.
10 bispecific antibody companies are profiled in the report
The total market value of bispecific antibody market is $5,508.3 million in 2022 .
The forecast period in the report is from 2023 to 2032.
High cost of bispecific antibody drugs is restraining factor for bispecific antibody market.
Loading Table Of Content...
Loading Research Methodology...



