Core Clinical Molecular Diagnostics Market Research, 2032
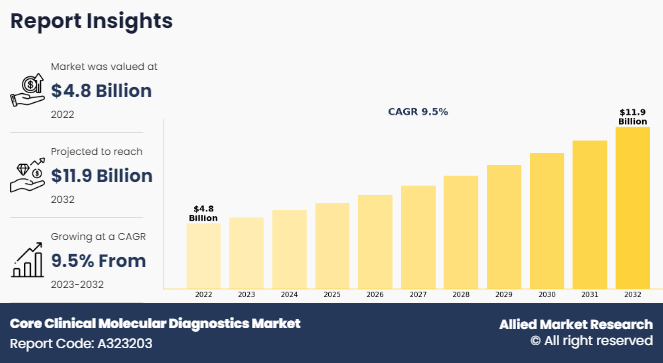
The global core clinical molecular diagnostics market size was valued at $4.8 billion in 2022, and is projected to reach $11.9 billion by 2032, growing at a CAGR of 9.5% from 2023 to 2032.The growing geriatric population serves as a pivotal driver for the robust growth of the core clinical molecular diagnostics market. The World Health Organization (WHO) projects a substantial increase in the global elderly population, with an estimated 1.4 billion people aged 60 years and over by 2030. This demographic shift underscores the escalating prevalence of infectious and oncological disorders. Responding to this surge, the increased demand for advanced diagnostic technologies is capable of detecting diseases at their molecular level.
Core clinical molecular diagnostics encompass vital laboratory techniques used to analyze genetic material (DNA, RNA) and proteins at the molecular level. These techniques, including Polymerase Chain Reaction (PCR), DNA sequencing, and Next-Generation Sequencing (NGS), are integral to disease diagnosis, prognosis, and treatment selection in personalized medicine. By identifying genetic variations, infectious agents, and biomarkers associated with diseases, core clinical molecular diagnostics facilitate tailored patient care and contribute to improved clinical outcomes.
Key Takeaways
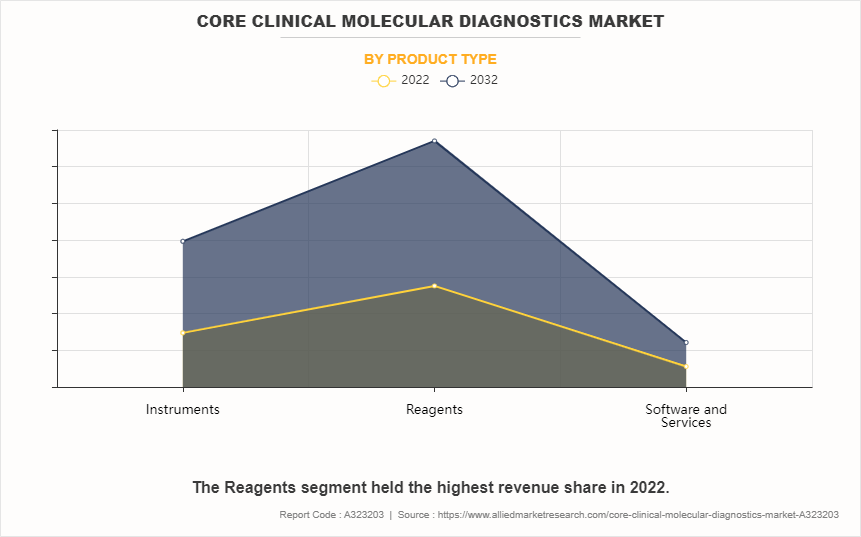
- On the basis of product type, the reagent segment dominated the core clinical molecular diagnostics market size in terms of revenue in 2022. However, the instruments segment is anticipated to grow at the fastest CAGR during the forecast period.
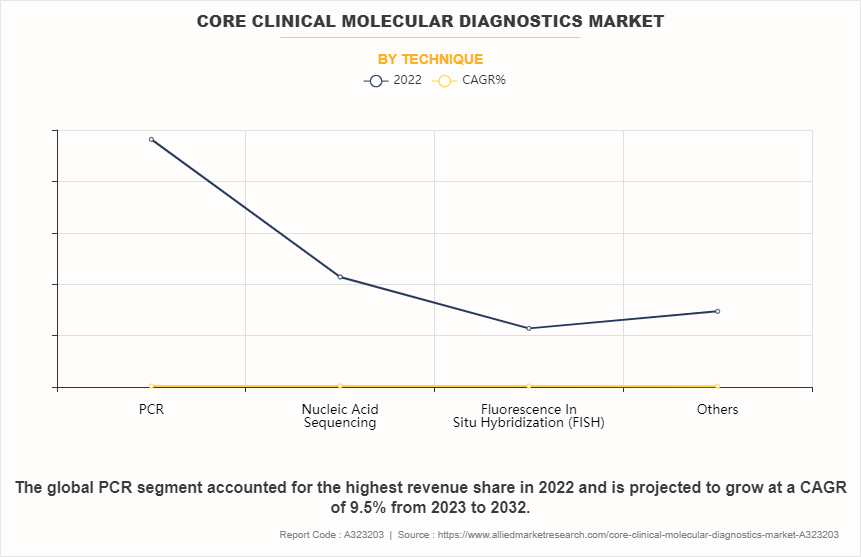
- On the basis of technique, the PCR segment dominated the core clinical molecular diagnostics market size in terms of revenue in 2022. However, the nucleic acid sequencing segment is anticipated to grow at the fastest CAGR during the forecast period.
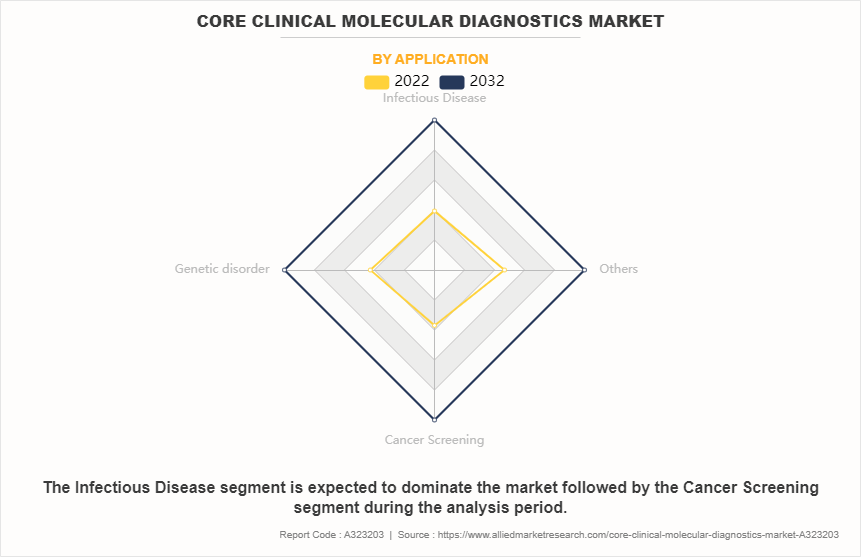
- On the basis of application, the infectious disease segment dominated the market in terms of revenue in 2022. However, the cancer screening segment is anticipated to grow at the fastest CAGR during the forecast period.
- Region wise, North America generated the largest revenue in 2022. However, Asia-Pacific is anticipated to grow at the highest CAGR during the forecast period.
Market Dynamics
The increasing prevalence of diseases, both chronic and infectious, is a significant driver for the growing demand for molecular diagnostic tests worldwide. Chronic conditions such as cardiovascular diseases, diabetes, and cancer are on the rise due to factors such as sedentary lifestyles, poor dietary habits, and aging populations. According to estimations by Globocan in 2020, around 19.2 million new cases of cancer are expected to be diagnosed by 2030, and it is projected that there will be 26 million new cancer cases across the world. Core clinical molecular diagnostic tests play a crucial role in accurately diagnosing these conditions by detecting genetic variations, pathogens, and biomarkers associated with specific diseases. Their ability to provide rapid, sensitive, and specific results enables timely intervention and personalized treatment strategies, contributing to improved patient outcomes and more effective disease management in both clinical and public health settings.
Moreover, rising healthcare expenditure is a significant driver of growth in the core clinical molecular diagnostics market. As healthcare spending increases globally, there is greater investment in advanced diagnostic technologies, including molecular diagnostics. Healthcare providers prioritize accurate and timely diagnosis to optimize patient care and outcomes. This trend is particularly pronounced in regions with aging populations and higher disease burdens. Consequently, the demand for core clinical molecular diagnostics continues to grow and is supported by increased healthcare funding and investment in innovative diagnostic solutions.
Furthermore, technological advancements are further anticipated to drive the growth of the core clinical molecular diagnostics market. Ongoing innovations, such as improved sequencing techniques, enhanced sensitivity of detection methods, and development of multiplex assays, enable more accurate and efficient molecular testing. These technological advancements not only increase the speed and accuracy of diagnosis but also expand the range of conditions that can be detected and monitored using molecular diagnostics. Furthermore, innovations in automation and miniaturization streamline laboratory workflows, reducing turnaround times and labor costs. As a result, healthcare providers are increasingly adopting molecular diagnostic technologies, driving market growth and improving patient care outcomes.
However, the high initial investment cost and limitations in accessibility and infrastructure in certain regions are anticipated to hinder the growth of the core clinical molecular diagnostics market. While advanced molecular diagnostic technologies offer significant benefits, their cost may be prohibitive for some healthcare providers. Additionally, inadequate infrastructure and limited access to these technologies in certain regions pose barriers to adoption. Addressing these challenges requires efforts to reduce costs, improve infrastructure, and enhance accessibility, ensuring equitable access to core molecular diagnostic technologies and driving market growth.
Moreover, regulatory support and increased awareness and screening programs are further driving the growth of the core clinical molecular diagnostics market. Regulatory bodies establish guidelines and standards to ensure the quality, safety, and efficacy of molecular diagnostic tests, fostering trust among healthcare providers and patients. This support encourages the development and adoption of innovative diagnostic technologies. Additionally, growth in awareness of the importance of early disease detection coupled with expanded screening programs drive the demand for molecular diagnostic tests and thereby also drives the core clinical molecular diagnostics market growth. These initiatives empower individuals to take proactive steps towards managing their health, resulting in increased utilization of molecular diagnostics and thereby driving the market growth.
Segments Overview
The core clinical molecular diagnostics market is segmented on the basis of product type, technique, application, and region. On the basis of product type, the market is segmented into Instruments, reagents and software & services. On the basis of technique, the market is segmented into PCR, nucleic acid sequencing, fluorescence in situ hybridization (fish), and others. On the basis of application, the market is segmented into infectious diseases, genetic disorders, cancer screening, and others. Region-wise, the market is analyzed across North America (U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and rest of Europe), Asia-Pacific (China, Japan, India, Australia, South Korea, and rest of Asia-Pacific), and LA (Brazil, Colombia, Argentina, and rest of LA) and MEA (GCC, South Africa, North Africa and rest of MEA).
By Product Type
Depending on product type, the market is segmented into instruments, reagents, and software & services. The reagents segment accounted for the largest core clinical molecular diagnostics market share in terms of revenue in 2022 and is expected to maintain its lead during the forecast period. This is attributed to its indispensable role in molecular diagnostics. Reagents are essential components in core clinical molecular diagnostics. Furthermore, innovation and adoption of advanced reagents in effective diagnostic workflows drive the segment growth. However, the instruments segment is expected to exhibit the fastest CAGR during the forecast period. This is primarily attributed to technological advancements and increasing demand for automated and high-throughput diagnostic platforms. These instruments enhance efficiency, accuracy, and throughput in molecular diagnostics, meeting the growing needs of laboratories for scalable and streamlined testing processes.
By Technique
Depending on technique, the market is segmented into PCR, nucleic acid sequencing, fluorescence in situ hybridization (FISH) and others. The PCR segment accounted for the largest core clinical molecular diagnostics market share in terms of revenue in 2022 and is expected to maintain its lead during the forecast period. PCR is extensively employed due to its versatility, sensitivity, and specificity in identifying genetic variations, pathogens, and disease-related mutations. Furthermore, the availability and continual advancements such as real-time PCR and digital PCR drive its growth in core clinical molecular diagnostic market. However, the nucleic acid sequencing segment is expected to exhibit the fastest CAGR during the forecast period. This is attributed to advancements in sequencing technologies, which have reduced costs and increased throughput, making whole-genome and targeted sequencing more accessible for research and clinical applications. Additionally, the ability of sequencing to provide comprehensive genetic information makes it invaluable for diagnosing genetic disorders, monitoring disease progression, and guiding personalized treatment strategies.
By Application
Depending on application, the market is segmented into infectious diseases, genetic disorders, cancer screening, and others. The infectious disease segment accounted for the largest share in terms of revenue in 2022 and is expected to maintain its lead during the forecast period. This is fueled by the growing global prevalence of infectious diseases, which increases the demand for diagnostic tests. Furthermore, ongoing outbreaks emphasize the necessity for rapid and precise diagnostics, contributing to continued market expansion within this segment.
However, the cancer screening segment is expected to exhibit the fastest CAGR during the core clinical molecular diagnostics market forecast period. The increased awareness and adoption for early cancer detection are owing to expanding screening programs worldwide, technological advancements in screening techniques enhancing accuracy and accessibility, and an aging population leading to a higher prevalence of cancer. Additionally, innovations in liquid biopsy and genetic screening contribute to the segment's growth by offering less invasive and more personalized screening options.
By Region
Region wise, the core clinical molecular diagnostics market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. North America had the largest share in terms of revenue in 2022 and is expected to maintain its lead during the forecast period owing to a well-established healthcare infrastructure, high healthcare spending, extensive adoption of advanced diagnostic technologies, robust research and development activities, and favorable government initiatives supporting healthcare innovation. Additionally, the region's large population base and increasing prevalence of chronic diseases further contribute to the continued growth of the diagnostic market.
However, Asia-Pacific is expected to exhibit the fastest growth during the forecast period, owing to several key factors. The region's robust economic development, coupled with a rapidly expanding population, is contributing to an increase in the number of patients suffering from various infectious diseases & chronic. Rising healthcare awareness and improving healthcare infrastructure in countries across Asia-Pacific are fostering greater access to core clinical molecular diagnostics.
Competitive Analysis
Major players such as F. Hoffmann-La Roche Ltd, and Qiagen have adopted product launch, acquisition, product development, product launch, and product approval as key developmental strategies to improve the product portfolio of the core clinical molecular diagnostics industry. For instance, in November 2021, F. Hoffmann-La Roche Ltd. announced the launch of the Cobas 5800 System, a new molecular instrument, in countries accepting the CE mark. Testing is one of the first lines of defense to protect a patients general well-being and is vitally important in quickly guiding their treatment. The Cobas 5800 System helps address challenges that laboratories are facing from an increase in patient testing, reimbursement complexities and the need for a more diverse testing options while providing meaningful and timely results.
Recent Developments in Core Clinical Molecular Diagnostics Industry
- In October 2020, Cepheid (Subsidiary of Danaher Corporation) announced that it has received clearance from the U.S. Food and Drug Administration for Xpert BCR-ABL Ultra for monitoring disease burden in patients with Chronic Myeloid Leukemia (CML).
- In May 2021, Qiagen announced the launch of an expanded scope of companion diagnostic (CDx) claims for the therascreen KRAS RGQ PCR Kit (therascreen KRAS Kit) after it received the U.S. regulatory approval as a companion diagnostic to aid in the identification of non-small cell lung cancer (NSCLC) patients.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the core clinical molecular diagnostics market analysis from 2022 to 2032 to identify the prevailing core clinical molecular diagnostics market opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the core clinical molecular diagnostics market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global core clinical molecular diagnostics market trends, key players, market segments, application areas, and market growth strategies.
Core Clinical Molecular Diagnostics Market Report Highlights
| Aspects | Details |
| Forecast period | 2022 - 2032 |
| Report Pages | 257 |
| By Product type |
|
| By Technique |
|
| By Application |
|
| By Region |
|
| Key Market Players | Qiagen NV., Abbott Laboratories, Becton Dickinson & Company, Agilent Technologies, Inc., Novartis AG, BioMerieux SA, Siemens Healthineers AG, cepheid ab, F. Hoffmann La Roche, Hologic, Inc. |
Analyst Review
The core clinical molecular diagnostics market highlights sustained growth driven by growth in population, increased prevalence of infectious, cancer and technological advancements. In addition, rise in awareness, early diagnosis, and shift in healthcare landscape toward patient-centric approaches are further anticipated to drive the market growth. Furthermore, government initiatives, supportive regulatory frameworks, and improvement in healthcare infrastructure contribute to the market's positive trajectory. With a strong emphasis on personalized medicine and an increasing focus on lifestyle-related risk factors, the core clinical molecular diagnostics market is positioned for sustained growth.
Furthermore, North America is expected to remain dominant during the forecast period, due to a surge in the use of core clinical molecular diagnostics. This is attributed to a well-established healthcare infrastructure and high healthcare expenditure. Furthermore, the presence of major market players fosters innovation and product development. In addition, Asia-Pacific and LAMEA are expected to offer lucrative opportunities to the key players, due to surge in awareness toward preventative healthcare.
The total market value of core clinical molecular diagnostics market is $4.8 billion in 2022.
The forecast period for core clinical molecular diagnostics market is 2023 to 2032
The market value of core clinical molecular diagnostics market in 2032 is $11.9 billion
The base year is 2022 in core clinical molecular diagnostics market .
Top companies such as F. Hoffmann-La Roche Ltd., Novartis, Abbott Laboratories, Becton, Dickinson and Company held a high market position in 202
2. These key players held a high market postion owing to the strong geographical foothold in North America, Europe, Asia-Pacific, LA and MEA.
The reagents segment is the most influencing segment in core clinical molecular diagnostics market. This is attributed to its indispensable role in molecular diagnostics. Reagents are essential components in PCR, sequencing, and other techniques, thus driving demand. Furthermore, rise in innovation and adoption of advanced reagents in effective diagnostic workflows drives the segment growth.
The major factor that fuels the growth of the core clinical molecular diagnostics market are increasing demand for precision medicine, rising prevalence of chronic diseases, and advancements in molecular diagnostics technology .
Core clinical molecular diagnostics refers to the essential techniques and procedures used in medical laboratories to analyze DNA, RNA, and proteins for the purpose of diagnosing genetic disorders, infectious diseases, and other conditions at the molecular level. These diagnostics play a crucial role in personalized medicine, as they allow healthcare professionals to tailor treatments to the specific genetic makeup of individual patients.
Loading Table Of Content...
Loading Research Methodology...



