Syphilis Immunoassay Diagnostics Market Research, 2031
The global syphilis immunoassay diagnostics market size was valued at $315.5 million in 2021, and is projected to reach $446.0 million by 2031, growing at a CAGR of 3.5% from 2022 to 2031. Syphilis is a serious bacterial infection that can affect both men and women. It is also seen in HIV-positive patients. Syphilis has several clinical manifestations, making laboratory testing a very important aspect of diagnosis. Syphilis diagnosis must always rely on a high level of clinical suspicion and routine screening for high-risk patients. The recent availability of faster, automated treponemal testing has resulted in a reversal of the normal syphilis screening strategy.

Blood tests can be used to diagnose syphilis. These tests can prove the presence of antibodies produced by the body to combat infections. As these antibodies reside in the body for years, the test can be used to establish whether an infection is current or old. Antibiotics are used to treat it, especially in the early stages.
Market Dynamics
The growth of the Syphilis Immunoassay Diagnostics Market Size is driven by rising in socioeconomic factors such as sexually transmitted diseases and an increase in the proportion of sex under the influence of numerous drugs such as heroin, cocaine, methamphetamine, cannabis, benzodiazepines, and alcohol. Moreover, the increase in rates of fatal syphilis infections, the surge in testing facilities, the widespread use of technology in testing, and the availability of on-the-spot testing help in the Syphilis Immunoassay Diagnostics Market Growth.
For instance, according to the data from World Health Organization (WHO), there were an estimated 374 million new infections with 1 of 4 STIs such as chlamydia (129 million), gonorrhea (82 million), syphilis (7.1 million), and trichomoniasis (156 million) in 2020. However, a lack of knowledge among individuals in emerging economies is expected to hinder the growth of this market. Conversely, the high growth potential in untapped emerging economies and advancements in the Syphilis Immunoassay Diagnostics Industry drives the Syphilis Immunoassay Diagnostics Market Size.
The global syphilis immunoassay diagnostics market is segmented based on product type, technology, end-user, and region. As per product type, the market is bifurcated into analyzers and kits & reagents. According to technology, it is classified into CLIA and ELISA. Depending on the end user, it is classified into blood banks, diagnostic labs, and hospitals. Region-wise, the market is studied across North America (the U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and the rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
Segments Overview
The syphilis immunoassay diagnostics market is segmented into Product Type, Technology, and End User.
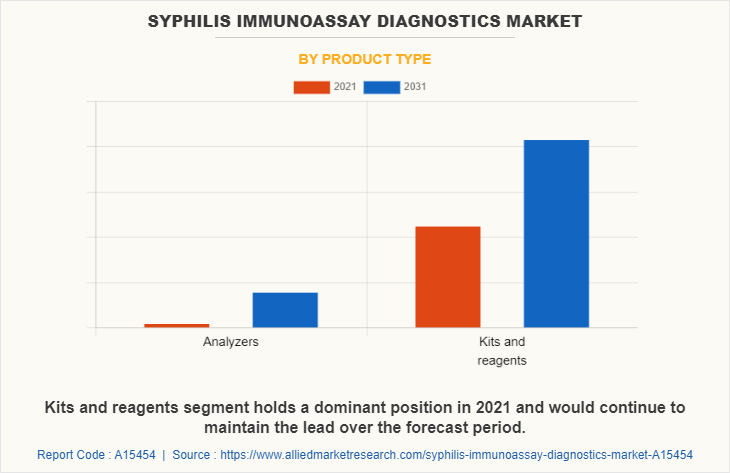
By Product Type
By product type, the market is classified into analyzers and kits & reagents. The kits and reagents segment is projected to exhibit the fastest market growth during the forecast period, owing to an increase in the prevalence of syphilis among the population, an increase in healthcare expenditure, and a rise in the adoption of syphilis diagnostic products for screening of the diseases. The syphilis diagnostic kits and reagents detect the presence of antibodies IgG and IgM to Treponema Pallidum (TP) in (whole blood), serum, or plasma samples to aid in the diagnosis of Syphilis. The increase in the prevalence of syphilis may trigger diagnostic procedures thereby increasing the adoption of diagnostic kits and reagents.
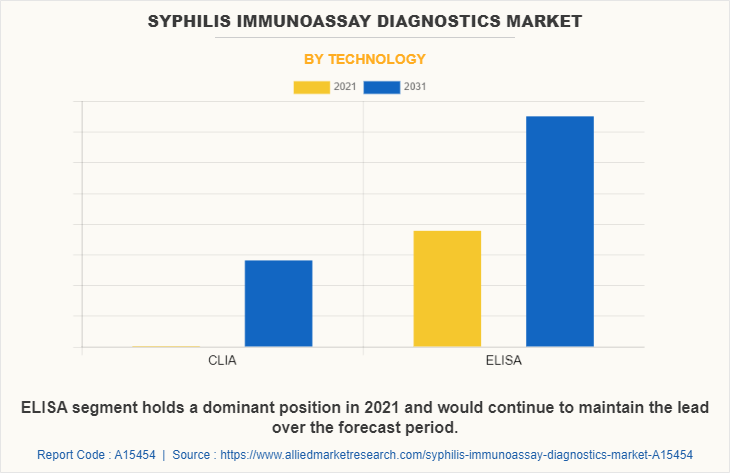
By Technology
By technology, the market is classified into CLIA and ELISA. The CLIA segment is anticipated to have largest Syphilis Immunoassay Diagnostics Market Share for the Syphilis Immunoassay Diagnostics Market Forecast period, owing to an upsurge in the number of patients of sexually transmitted diseases, an increase in the number of syphilis diagnosis procedures, and a rise in the number of research and development activities for syphilis testing. CLIA can be employed for screening syphilis in blood donors for its good sensitivity, ease of automation and short turn-around-time. Hence, the increased adoption of this technology in the screening can help the growth of the market. Moreover, the surge in testing facilities, the widespread use of technology in testing, and the availability of on-the-spot testing help in the growth of the market.
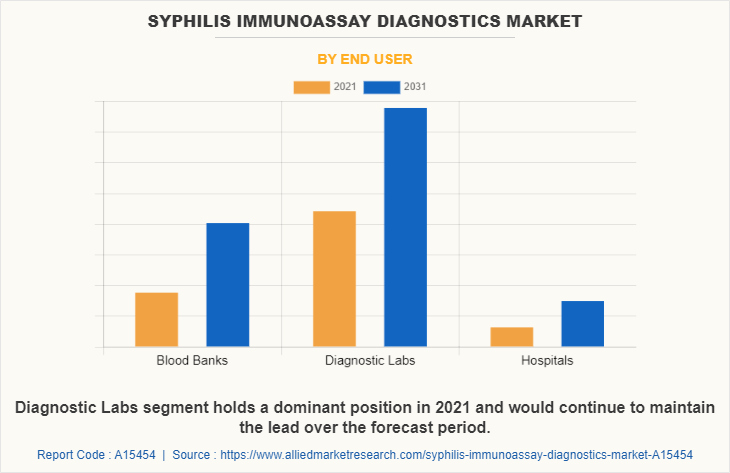
By End User
By end user, the market is classified into blood banks, diagnostic labs, and hospitals. The blood banks segment is anticipated to have the largest Syphilis Immunoassay Diagnostics Market Share, owing to an increase the in the number of biotechnology companies, growth in the Syphilis Immunoassay Diagnostics Industry, and a rise in research activities of cell therapy. Moreover, the surge the in the number of pharmaceutical companies further contributed to the growth of the market. The U.S. Food and Drug Administration (FDA) recommends that donation facilities are required to perform a serological screening test on each donation of blood for the presence of syphilis. The donors must be tested upon their first plasmapheresis and then every four months thereafter as they do not fail the first test in the blood banks.
By Region
In 2021, North America was the dominant region and is expected to remain dominant during the forecast period, owing to high prevalence rate of syphilis, an increase the in the number of market players, and a surge in testing facilities in the region. However, Asia-Pacific is expected to witness the highest CAGR during the forecast period, owing to the presence of high populace countries, such as India and China, which in turn increases the prevalence rate of sexually transmitted disease and the increasing number of strategies and trends adopted by the market players.
The list of key players profiled are Abbott Laboratories, Becton, Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories, Inc, Danaher Corporation, DiaSorin Group, F. Hoffmann-La Roche AG, Quidel Corporation, Siemens AG and Shenzhen New Industries Biomedical Engineering Co., Ltd.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the syphilis immunoassay diagnostics market analysis from 2021 to 2031 to identify the prevailing Syphilis Immunoassay Diagnostics Market Opportunity.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the syphilis immunoassay diagnostics market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global syphilis immunoassay diagnostics market trends, key players, market segments, application areas, and market growth strategies.
Syphilis Immunoassay Diagnostics Market Report Highlights
| Aspects | Details |
| Market Size By 2031 | USD 446 million |
| Growth Rate | CAGR of 3.5% |
| Forecast period | 2021 - 2031 |
| Report Pages | 247 |
| By Product Type |
|
| By Technology |
|
| By End User |
|
| By Region |
|
| Key Market Players | Fujirebio, Abbott Laboratories, BECTON DICKINSON & COMPANY, Diasorin S.P.A, BioRad Laboratories Inc, , F. Hoffmann-La Roche AG, Shenzhen New Industries Biomedical Engineering Co. Ltd, Danaher Corporation, Siemens Healthineers AG, , BioMerieux SA |
Analyst Review
In accordance to several interviews conducted, syphilis has diverse clinical manifestations and shares many clinical features with other treponemal and nontreponemal diseases. The serological tests fall into two categories namely nontreponemal tests for screening and treponemal tests for confirmation. All nontreponemal tests measure both immunoglobulin (Ig) G and IgM antiphospholipid antibodies formed by the host in response to lipoidal material released by damaged host cells early in infection and lipid from the cell surfaces of the treponeme itself.
Asia-Pacific is expected to witness the highest CAGR during the analysis period, owing to presence of high populace countries such as India and China, which in turn increases the prevalence rate of syphilis, increase in awareness about syphilis immunoassay diagnostics, and increase in government expenditure on healthcare & other trends adopted by the market players.
The total market value of the Syphilis Immunoassay Diagnostics market is $315.5 million in 2021.
The forecast period in the report is from 2022 to 2031.
North America is the largest regional market for Syphilis Immunoassay Diagnostics.
The market value of the Syphilis Immunoassay Diagnostics market in 2022 was $327.4 million.
The top companies that hold the market share in Syphilis Immunoassay Diagnostics market are Abbott Laboratories, Becton, Dickinson and CompanybioMérieux SA, Bio-Rad Laboratories, Inc., Danaher Corporation, DiaSorin Group, F. Hoffmann-La Roche AG, Quidel Corporation, Siemens AG and Shenzhen New Industries Biomedical Engineering Co., Ltd.
The base year for the report is 2021.
Yes, Syphilis Immunoassay Diagnostics companies are profiled in the report
The key trends in the Syphilis Immunoassay Diagnostics market are by an increase in the number of hospitals, advancements in the Syphilis Immunoassay Diagnostics and R&D in the Syphilis Immunoassay Diagnostics treatment.
Loading Table Of Content...



